Management of anterior instability of the shoulder with bone defects
Manejo de la inestabilidad anterior de hombro con defectos óseos
Resumen:
Objetivo: el objetivo del presente artículo de revisión es resumir la información más relevante acerca de la inestabilidad de hombro con defectos óseos y servir de guía para el estudio de esta patología y su manejo.
Método: revisión de la literatura.
Resultado y conclusiones: el estudio de la inestabilidad con defecto óseo implica una correcta detección clínica y radiológica. La tomografía computarizada con reconstrucción 3D puede proporcionar un análisis preoperatorio objetivo de la ubicación y el grado del defecto, y la artroscopia diagnóstica juega un papel importante en la toma de decisiones de tratamiento. La definición de los defectos óseos de la glena, defectos humerales y los defectos óseos combinados, conjuntamente con el concepto del glenoid track, son fundamentales para la adecuada comprensión del riesgo de recidiva y la posición relativa glenohumeral para que esta se produzca, justificando así el uso de técnicas de bloque óseo asociadas a la reparación de tejidos blandos. Es objeto de debate el porcentaje de defecto de la glena crítico para justificar la necesidad de aplicar estas técnicas. El procedimiento de Latarjet y otras técnicas de bloque óseo se usan ampliamente para tratar defectos óseos glenoideos. Para los defectos óseos humerales, el remplissage es una técnica ampliamente utilizada, destacando el conocimiento de la posible influencia que puede tener sobre el rango de movimiento postoperatorio.
Relevancia clínica: las recomendaciones planteadas en este artículo pretenden servir de soporte para el estudio y la toma de decisiones en la práctica clínica de especialistas que se enfrenten a esta patología.
Abstract:
Objective: A review is made to summarize the most relevant information on shoulder instability with bone defects and to serve as a guide for the study of this type of disorder and its management.
Method: A literature review was carried out.
Results and conclusions: The study of instability with bone defects requires correct clinical and radiological detection. Computed tomography with three-dimensional reconstruction can offer an objective preoperative analysis of the location and degree of the defect, and diagnostic arthroscopy plays an important role in deciding treatment. The definition of bone defects of the glenoid cavity, humeral defects and combined bone defects, together with the glenoid track concept, are fundamental for a correct understanding of the risk of relapse and the relative glenohumeral position in which the latter occurs - thus warranting the use of bone block techniques associated to soft tissue repair. The critical percentage glenoid cavity defect justifying the need to apply these techniques is subject to debate. The Latarjet procedure and other bone block techniques are widely used to treat glenoid bone defects. In the case of humeral bone defects, remplissage is a widely used procedure, and may exert an influence upon the postoperative range of motion.
Clinical relevance: The recommendations made in this article seek to serve as a support for study and decision making in the clinical practice of specialists that deal with disorders of this kind.
Introduction
Coordinated interaction among static, dynamic and bone structures is necessary to keep the humeral head centred within the glenoid cavity in all spatial positions of the hand(1,2). Instability may be the result of disorders of both the static stabilizers (labrum, capsule and glenohumeral ligaments) and of the dynamic stabilizers (rotator cuff muscles) - with the consequent alteration of joint anatomy. Following an initial traumatic luxation (dislocation), the most common disorder is anteroinferior capsulolabral avulsion with or without attenuation of the corresponding associated glenohumeral ligament(3,4).
A number of factors have been reported to affect the recurrence rates, including the age of the patient, the type of dislocation involved, contact sports, hyperlaxity and significant bone defects(5,6). Bone defects due to posterosuperior impacting of the humeral head or anteroinferior glenoid bone loss play an important role in anterior glenohumeral instability by altering the contact area of the joint, its congruence and the function of the static restriction components(7,8,9,10). The study of bone defects and their relation to instability has been widely addressed in the literature. Burkhart and De Beer(10) described the influence of bone defects in shoulder instability, and underscored their role in the failure of arthroscopic stabilization procedures, with the recording of a 67% relapse rate. They defined the engaging Hill-Sachs lesion, and recommended that patients with such lesions should not be treated with soft tissue arthroscopic techniques.
A number of studies allow us to understand glenoid and humeral bipolar bone defects and their implication in relapsing luxation. In this regard, mention must be made of the study published by Yamamoto et al.(11) and their glenoid track (GT) concept, relating the size and location of the glenoid and humeral defects and their implication in instability, based on analyses in cadavers. More recently, Di Giacomo, Itoi and Burkhart(12), with their description of Hill-Sachs lesions and their concept of the on-track/off-track lesion as an evolution of the known engaging/non-engaging lesion, have allowed us to understand these defects and assess them in the treatment decision making process of such lesions.
The management of bone defects in shoulder instability has been a challenge for surgeons for many years. It has been demonstrated that certain non-anatomical procedures prevent luxation relapse, but may be associated to stiffness and osteoarthrosis(13).
The present narrative literature review summarizes the most relevant information on anterior shoulder instability with associated bone defects and serves as a guide for the study of this type of disorder and its management.
Relevance of glenoid bone defects
Although glenoid defects are present in only 22% of all patients with acute luxation, they have been reported in 49-86% of all those with relapsing luxation(1,14,15,16,17,18). Glenoid bone loss and the reabsorption of fragments leads to alteration of the morphology of the glenoid cavity, acquiring an inverted pear shape(10,19,20). Anteroinferior deficiency of the glenoid bone alters the capacity to keep the humeral head centred within the joint range - this in turn leading to lessened resistance to anterior dislocation of the humeral head.
In the presence of a "significant" magnitude of defect, the humeral head can dislocate anteriorly with only a minimum amount of translation. There has been much debate on how to quantify anterior bone loss and on how much bone loss is required to regard a defect as being significant. Burkhart and DeBeer(10) recorded a recurrence rate of 4% after arthroscopic Bankart repair without the presence of significant bone defects (over 25% of loss of inferior glenoid diameter or engaging Hill-Sachs lesion) and a recurrent instability rate of 67% in athletes with significant defects (over 25% of loss of inferior glenoid diameter or engaging Hill-Sachs lesion).
Gerber and Nyffeler(21) in turn reported a more than 30% loss of resistance to anterior shoulder dislocation when the magnitude of the anteroinferior defect was greater than half of the maximum anteroposterior (AP) glenoid diameter. Itoi et al.(7) performed sequential osteotomies of the anteroinferior glenoid cavity in shoulders of cadavers and found stability on performing anterior translation to be significantly lower in the presence of a bone defect of over 21% of the diameter of the glenoid cavity. Other studies have also demonstrated that a glenoid defect of ≥ 25% of the glenoid width causes instability even after Bankart repair, while a defect of ≤ 17.5% does not cause instability(22). It has been shown that an increase in the size of the glenoid bone defect is correlated to a decrease in the stability of the glenohumeral joint(21,23), though agreement is lacking on the exact magnitude of bone loss required in order to regard a lesion as being significant.
A possible explanation for this lack of agreement is that these original biomechanical studies investigated isolated glenoid defects, while we now know that most patients with relapsing anterior luxation have combined defects of the humeral head and the glenoid cavity(24).
Other more recent biomechanical studies examining combined bone defects have recorded a significant decrease in glenohumeral stability in the presence of glenoid defects as small as 10-15% of the glenoid width(25,26).
More recently, the concept of subcritical defects has been established, in which a smaller percentage of bone loss after Bankart repair does not necessarily result in a new luxation episode, though it may produce a poorer patient functional outcome according to the clinical scales on comparing cases of exclusively soft tissue repair versus patients subjected to bone augmentation procedures. In this regard, it has been reported that defects larger than 13.5% lead to a clinically significant decrease in the Western Ontario Shoulder Instability (WOSI) score, consistent with unfavourable outcomes, even in patients that do not experience further luxation episodes(27).
Relevance of humeral bone defects
Hill-Sachs lesions imply a posterolateral humeral head impactation defect when the humeral head comes into contact with the anteroinferior glenoid margin during anterior dislocation of the shoulder. It is present in approximately 47% of the cases of a first anterior glenohumeral luxation and in up to 90% of all the recurrent cases(15), and a direct correlation is moreover observed between the severity of the bone defects and the number of relapsing luxation episodes(8,18,28).
Lesions corresponding to under 20% of the circumference of the humeral head generally do not cause significant instability(2,14). Lesions measuring over 40% in size are considered to be large and are associated to a greater probability of recurrent luxation(2,18). Defects of between 20-40% may be important, but this depends on their location, orientation and trajectory with respect to the anteroinferior glenoid cavity.
Not only the location of the Hill-Sachs lesion but also its orientation appear to influence the risk of recurrent shoulder dislocation. Burkhart and De Beer(10) define an engaging Hill-Sachs lesion as an injury in which the long axis of the Hill-Sachs lesion is parallel to the anterior glenoid cavity with the extremity in functional abduction and external rotation position. Although it is difficult to establish an adequate definition of the term "engaging", and its use can lead to error because there may be interobserver differences in its assessment by different surgeons, the authors reported that in the presence of such a lesion, repair procedures focused exclusively on the soft tissues present a high failure rate. They moreover explained that an engaging lesion is produced with the extremity in abduction and external rotation, while a non-engaging lesion is created with the extremity in adduction.
Cho et al.(29) quantified the orientation of the Hill-Sachs lesion measuring the Hill-Sachs angle between the longitudinal axis of the humeral diaphysis and the axis of the deepest portion of the Hill-Sachs lesion in the frontal plane, in the three-dimensional (3D) computed tomography (CT) scan. They found the mean Hill-Sachs angle to be 25.6º for engaging Hill-Sachs lesions versus 13.8º for non-engaging Hill-Sachs lesions.
Di Giacomo et al.(30) also measured the orientation of the Hill-Sachs lesion and divided their patients into two groups: those in which initial luxation occurs with the extremity at less than 60º of abduction (ADD group) and those in which it occurs with the extremity at more than 60º (ABD group). They found the Hill-Sachs angle to be 32.4º in the ABD group and 16.1º in the ADD group. In their series, the authors speculated that the Hill-Sachs lesion in the ABD group was more likely to present engagement because it was more parallel to the anterior glenoid cavity with the extremity in functional position. The orientation of the Hill-Sachs lesion was determined by the position of the extremity in the moment of the injury, and engagement proved more likely if it was parallel to the glenoid cavity with the extremity in functional position.
In another biomechanical study, Kawakami et al.(31), in an attempt to specify the position of the extremity in the moment of the Hill-Sachs lesion, reported that the position of the arm where the Hill-Sachs lesion and the anterior glenoid margin adjust best is at 74º abduction, 27º external rotation and 3º horizontal flexion, which appears to be the position when the Hill-Sachs lesion occurred. Two possibilities were suggested on the basis of these results: luxation occurred in this position or the Hill-Sachs lesion was not produced at the time of the luxation but later within the range of motion. Hence, this study affords improved understanding of the moment in which the Hill-Sachs lesion occurs.
Combined defects and glenoid track concept
Bone lesions of the glenoid cavity and humerus are very common in patients with anterior shoulder instability. Approximately 80% of them present both lesions at the same time - a situation known as a bipolar lesion. As has been mentioned, biomechanical studies have demonstrated that a glenoid defect of ≥ 25% of the glenoid width causes instability even after Bankart repair, while a defect of ≤ 17.5% does not usually cause instability(32). The range between 17.5-25% has been referred to as the "grey zone", known as subcritical bone loss(33). The risk of causing instability in a Hill-Sachs lesion is dependent not only upon the lesion itself but also on the size of the glenoid cavity defect. The glenoid track (GT) concept was introduced to globally evaluate these lesions.
Yamamoto et al.(34), in their study in cadavers, found that with the arm in maximum external rotation, horizontal extension and with incremental elevation of the extremity (0º, 30º and 60º abduction), contact of the glenoid cavity changes from the inferomedial portion to the superolateral part of the posterior portion of the humeral head, creating a contact zone which they called the glenoid track. The medial margin of the GT is located 18.4 ± 2.5 mm medial to the footprint of the rotator cuff, which is equivalent to 84 ± 14% of the diameter of the glenoid cavity. The authors thus found that a Hill-Sachs lesion is more likely to present engagement if it extends over the medial margin of the GT.
The width of the GT decreases if there is a glenoid bone defect. Thus, glenoid and humeral head bone defects can be evaluated one against the other. If the medial margin of a Hill-Sachs lesion lies within the GT, there is bone support adjacent to the Hill-Sachs lesion, and the latter will be on-track. However, if the medial margin of the Hill-Sachs lesion lies more medial than the GT, there is no bone support, and the Hill-Sachs lesion will be off-track(12). The method for calculating the GT is clearly defined in the following section.
Evaluation of the bone defects
Radiological evaluation
The bone defect can be evaluated from plain radiographs or CT or magnetic resonance imaging (MRI) scans, or intraoperatively through arthroscopy.
The radiological study includes an anteroposterior (AP) view and a lateral view of the scapula and axilla that confirm the direction of the luxation and the associated bone defects or fractures. The Stryker notch view highlights the posterolateral zone of the humeral head, being able to identify bone defects at this level, and the West Point view in turn specifically assesses glenoid bone loss, as reported by Itoi et al.(35) in their study, where a close correlation was observed with the CT findings in estimating the glenoid bone defect.
Computed tomography is the imaging technique of choice for evaluating bone deficiencies. The indications of CT include significant patient apprehension at minor abduction levels, instability with minimum provocation, multiple dislocation episodes, revision surgery and any bone defect detected by the plain radiographs. Different measurement methods are available for calculating the real size of the glenoid cavity and of the bone defect, based on preoperative two-dimensional (2D) and 3D CT scans(16,36), where digital subtraction of the humeral head is performed to obtain a complete frontal view of the glenoid cavity(1,37,38).
The anterior glenoid margin has a curved morphology; at the time when bone loss begins to manifest, the curve straightens, and this is the first sign of glenoid bone loss. As the bone defect progresses, the anterior straight line is lengthened, and when severe degrees of bone loss are present, the anterior curve of the glenoid cavity becomes concave(36). Comparison of the images of the affected glenoid cavity versus the contralateral cavity is the most precise method for calculating the glenoid bone defect, since there is almost perfect symmetry between the shape and size of the two glenoid fossae, provided the contralateral glenoid cavity has not suffered glenohumeral luxation episodes(39,40).
In order to calculate the defect, we must obtain a frontal view of the glenoid cavity and trace two lines. The first represents the reference line and is traced following the long axis of the glenoid cavity from the supraglenoid tubercle to the lowermost part of the glenoid cavity. The second line is traced at a right angle to the long axis of the glenoid cavity in the lower half, at the point corresponding to the maximum width of the glenoid surface. The same lines are then traced in the contralateral shoulder to use the healthy glenoid cavity as a guide to size and morphology, allowing us to precisely determine the degree of bone loss(41).
Magnetic resonance imaging has also been described as a tool for measuring glenoid bone defects, allowing lesser patient irradiation and the study of associated lesions. The most widely used measurement method is that published by Sugaya et al., where the diameter of the glenoid cavity is measured using the best fit circle, tracing a circle that adapts to the circumference of the glenoid cavity and adjusting it at the lower margin(42). However, the estimation of bone loss at the lower part of the glenoid cavity based on the posterior margin may overestimate the defect by up to 5%, since there are differences between the anterior and posterior contour of the glenoid cavity - overestimation being greater if the bone defect is moderate or severe(43). Despite the above, it has been reported that such quantification compares favourably with the measurements obtained with CT and 3D CT; its precision is related to the skill of the operator, however, since there is a learning curve for mastering the technique(44).
A number of methods are currently available for evaluating and quantifying Hill-Sachs lesions. Measurement of length, width and depth in CT reconstructions has shown strong inter- and intra-observer correlation coefficients in the literature(45). It has been reported that lesions which are larger and are oriented more perpendicular to the humeral axis are more likely to be engaging lesions(29). Three-dimensional CT is the gold standard for evaluating Hill-Sachs lesions. However, some studies have reported no significant differences between 3D CT and 3D MRI, thus indicating that the patient radiation exposure associated to CT could be avoided(46).
Hill-Sachs deformity is best measured in axial views at the uppermost surface of the proximal humerus, above the coracoid process(47). The widest region of the Hill-Sachs deformity is the most important measurement with respect to the glenoid track, and the depth of the deformity can be studied by tracing a circle through the upper surface of the proximal humerus(41).
Calculation of the GT allows us to determine the probability of engagement of the Hill-Sachs lesion at the anteroinferior margin of the glenoid cavity, and is a useful tool when it comes to planning surgery(45,48). Its determination is important, among other reasons because it may modify the choice of treatment and the type of surgery. In this regard, in the case of off-track lesions, it may be necessary to add the remplissage technique or bone stop processes, depending on the size of the glenoid defect and the risk of recurrence.
Calculation of the glenoid track
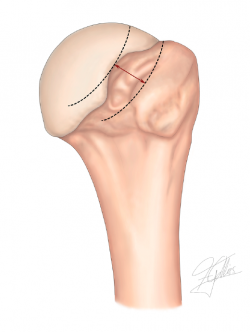
In order to classify a Hill-Sachs lesion as on-track or off-track, we need to follow a series of steps (Figures 1 and 2):
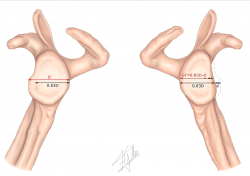
1. Measurement of the real glenoid cavity diameter (D).
2. Measurement of the glenoid bone defect (d).
The measurements previously described in the CT scan can be used to calculate these parameters, comparing them against the contralateral shoulder, or alternatively we can measure the best fit circle in the sagittal view of the MRI scan.
3. Calculation of GT using the following formula: 0.83 × (D − d).
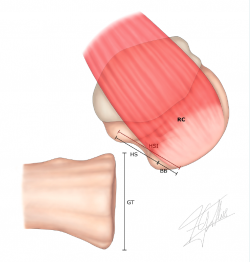
4. Measurement of the Hill-Sachs interval (HSI) (Figure 3): this is the result of measuring the Hill-Sachs width (HS) plus the bone bridge (BB) between the medial margin of the insertion of the cuff and the lateral region of the Hill-Sachs lesion(49) (HSI = HS + BB).
After performing the abovementioned calculations, we can find two situations:
1. GT > HSI: on-track lesion. The entire lesion lies within the GT, with no "engagement" in the glenoid cavity on performing the most extreme movements.
2. GT < HSI: off-track lesion. Part of the lesion lies outside the GT, engaging at the anteroinferior margin of the glenoid cavity on performing the most extreme movements.
Although strong inter-observer agreement has been observed (> 90%) in evaluating the glenoid defect, some publications indicate that there may be important variability in using the medial margin of the insertion of the cuff and the margins of the Hill-Sachs defect in calculating the Hall-Sachs interval, thereby conditioning the measurement of GT and the treatment algorithm(50,51). This variability is mainly evidenced in 2D images and is more manifest with MRI than with CT(40).
In an attempt to clarify the existence of subcritical bone defects in the Hill-Sachs lesion and their influence upon the results on the postoperative assessment scales, Yamamoto et al. divided the GT into four zones, based on the measurement of "Hill-Sachs occupancy" percentage on the 3D CT scan. The authors found that patients with on-track lesions can be divided into two subgroups: (i) those with Hill-Sachs occupancy percentage ≥ 75% (peripheral track lesions), showing significantly poorer functional outcomes even without further luxation episodes versus (ii) those with occupancy percentage < 75% (central track lesions)(52).
Arthroscopic evaluation
A significant glenohumeral bone defect can be identified during arthroscopy by looking for the "inverted pear" shape of the glenoid cavity (> 25% loss of inferior glenoid diameter) or evaluating the track of the Hill-Sachs lesion(10). During diagnostic arthroscopy, traction can be removed with evaluation of the range of motion with flexion, abduction and progressive external rotation. If the humeral head falls within the anteroinferior glenoid defect with the arm in abduction and external rotation, we have an engaging Hill-Sachs lesion or off-track bipolar lesion.
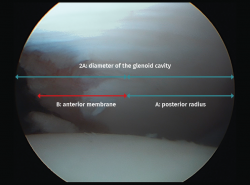
For arthroscopic evaluation of the glenoid bone defect, with the camera in the anterosuperior portal, we measure the distances from the posterior glenoid cavity to the denuded area, and from the denuded area to the anterior margin. At the joint border in the lower portion of the glenoid cavity, the anterior and posterior margins are equidistant from the denuded central area; consequently, to calculate the defect, it is assumed that this measures the difference between the two of them. In order to express the result as a percentage, we divide the defect by the double of the distance from the denuded area to the posterior margin(53) (Figure 4).
Techniques for management of the glenoid bone defect
(Table 1)
Bristow procedure
Described by Helfet in 1958 in the name of his teacher Rowley Bristow, from South Africa, this procedure uses the tip of the coracoid process, distal to the insertion of the pectoralis minor, thus leaving the tendon inserted in the transferred coracoid portion, passing it through an incision at subscapular level to place it in the lower part of the glenoid cavity(54). In this technique, the coracoid is placed orienting it through the surface where the osteotomy has been made. Helfet originally did not use the screw in his technique and only sutured the fragment to the anterior muscle wall. In 1964 the technique was modified by Mead and Sweeney(55) to include rigid internal fixation.
Latarjet procedure
This procedure is widely used to treat glenoid cavity bone defects in shoulder instability using open surgery, a mini-open technique or arthroscopic approach(56,57,58). In 1954, Latarjet(59) described the coracoid bone block technique, suggesting fixation with a screw of its horizontal portion flush with the anteroinferior margin of the glenoid cavity by means of a horizontal incision through the subscapularis muscle fibres. May(60) attributed the efficiency of the technique more to the hammock effect exerted by the coracobrachialis muscle tendon and the tendon of the subscapularis muscle in abduction - external rotation than to the bone stop itself.
Patte((61) proposed the term triple lock to describe the efficacy of the technique:
1. Bone effect, thanks to stable fixation with the coracoid bone block screw, flat over a subequatorial position and flush with the anterior margin of the glenoid cavity.
2. Hammock effect due to preservation of the muscle-tendon fibres of the lower third of the subscapularis muscle.
3. Capsule repair effect with suturing of the lateral capsular flap to the remaining coracoacromial ligament inserted in the coracoid bone.
This procedure is widely used, and the reported outcomes are very satisfactory(62). Burkhart et al.(9) presented the results of 102 patients treated with the modified Latarjet procedure for glenoid bone losses of over 25%. Four percent of the patients suffered recurrent luxation, 5% complained of instability, and there was a mean 5º loss of external rotation with the arm in adduction. Allain et al.(63) reported on 56 patients monitored during an average of 14 years after the Latarjet procedure. There were no luxation relapses, and 12% of the patients complained of recurrent instability. Hovelius et al.(64) described the long-term follow-up (> 10 years) of patients subjected to the Bristow-Latarjet procedure, with fusion of the coracoid bone in 83% of the cases, recurrent luxation in 5%, and revision surgery in 1%.
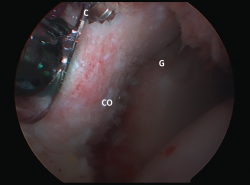
Satisfactory outcomes and great effectiveness have been reported with this procedure in soccer players(65) and rugby players(66). Good outcomes have also been described in successfully restoring shoulder stability in elderly patients(67) and when used in revision surgery(68). More recently, the results obtained with the arthroscopic Latarjet procedure (Figure 5) have been reported, with outcomes equivalent to those of the open procedure, though the laparoscopic approach is clearly more demanding from the technical perspective(57,69).
Isolated glenoid bone grafting techniques
These alternatives involve the use of iliac crest bone grafts(70) for reconstruction based on bone block techniques, as modifications of the Eden-Hybinette technique(71) or the J graft procedure(72), and reconstruction techniques using distal tibial grafts(73).
Eden-Hybinette technique
Described in 1918 by Eden and in 1932 by Hybinette, this technique uses an iliac crest graft for glenoid bone augmentation. With regard to its arthroscopic variant, first described by Taverna et al.(74), a number of modifications have been developed. One of them, described by Boileau et al.(75), is indicated as rescue treatment in patients with failed prior Latarjet surgery, based on the use of tricortical ipsilateral iliac crest grafts. After levelling of the cortical portion, a perforation is made with a 2.8-mm drill for passing the guiding sutures. The glenoid cavity is prepared using a specific guide and drilling to subsequently recover the bone block with guiding sutures. The graft is then fixed with the suspension system and anterior and posterior button in the glenoid cavity. The authors reported satisfactory results using clinical assessment scales, with no complications or need for repeat surgeries.
J graft procedure
This is an open technique involving the use of a J-shaped bicortical iliac crest graft that is impacted in the glenoid cavity in the zone of the defect, following preparation of the glenoid cavity. It has been described for fixation through impaction, with the exceptional use of additional fixation using a cannulated screw, where required. The authors reported satisfactory results using clinical assessment scales, as well as a loss of external rotation of 4.36º in adduction and 3.19º at 90º abduction. There were no recurrent instability cases and a single traumatic fracture of the graft(72).
The interest in eliminating metal implants for graft fixation has favoured the development of arthroscopic techniques that obviate their use. In addition to the mentioned J graft procedure, examples of these techniques are the method described by Hachem et al.(76), using cerclage with high-resistance sutures; the procedure described by Zhao et al.(77), with anchoring sutures in the native glenoid cavity; and the technique described by Whelan et al.(78), involving the use of acellular human dermal matrix, among other procedures.
Distal tibial graft
This procedure is described for relapsing instability with an anterior glenoid cavity bone defect of at least 15%, using distal tibial allografts to reconstruct the glenoid defect. Excellent clinical outcomes with minimal graft reabsorption have been reported over an average follow-up of 45 months(74).
Hill-Sachs lesion management
The guiding principle of Hill-Sachs lesion management is to avoid engagement with the anterior glenoid margin. This can be achieved using anatomical or non-anatomical techniques, filling the humeral head defect. Some of the described options involve shortening or re-tensing of the anterior soft tissue(79), or rotational osteotomy of the humerus(80). Among the defect filling alternatives, mention can be made of filling with bone graft material(81) or soft tissue (remplissage)(82), or percutaneous plasty/correction of the humeral head(83,84).
Remplissage
Connolly(85) was the first to describe tenodesis of the infraspinatus tendon in the humeral head defect, and posteriorly Wolf et al.(82) were the first to describe the arthroscopic variation of the technique, applied in combination with Bankart repair, and which they referred to as remplissage. The technique involves filling of the humeral head defect through capsulotenodesis of the infraspinatus tendon and glenohumeral capsule. The results of the original technique(86) and its modification(87) have been reported, and in view of their simplicity, these procedures have become popular and are widely used.
Since their initial description, excellent functional outcomes have been reported in application to recurrent instability, though with the inconvenience of a series of repercussions upon the postoperative range of motion. When compared with isolated Bankart repair, these procedures have been reported to avoid the 20% incidence of recurrences associated with the former technique, with no significant differences in terms of resulting motion restrictions(88,89).
Biomechanical studies such as that published by Elkinson et al.(90) have recorded a resulting limitation of external rotation in adduction and abduction of 14.5º and 6.2º, respectively. Boileau et al.(91) likewise reported restriction of external rotation after remplissage of 8º in adduction and 9º in abduction in their series, with a return to sports activity in 90% of the cases and restoration to pre-injury levels in 68%.
Humeral bone graft
Humeral head allografts, more commonly used in the context of large Hill-Sachs lesions due to instability, have afforded significant improvement in range of motion and in the American Shoulder and Elbow Surgeons (ASES) scores up to one year after the operation. The rates referred to return to work and patient satisfaction after the operation are high. However, the incidence of complications (20-30%) such as graft necrosis and reabsorption, and osteoarthrosis, as well as the reoperation rates (26.67%), are significant(81).
Therapeutic alternatives in the management of bone defects
Correct interpretation of the bone defect and of the GT, adding predictive factors (age, gender, sports activity(9), concomitant lesions, etc.) is necessary for adequate therapeutic decision making.
Balg and Boileau published the Instability Severity Index Score (ISIS) in an attempt to preoperatively predict success or failure with isolated soft tissue repair(92). This score is based on a preoperative questionnaire, clinical examination and radiographic review. Those patients with a score of over 6 points had a recurrence rate of 70% with soft tissue repair alone, and in these cases the authors recommend an added procedure to deal with the bone defect - isolated arthroscopic Bankart repair being insufficient.
The use of this assessment scale has been the subject of debate in the literature, and some authors have reported that in patients with anterior instability in which Bankart repair is contemplated with scores of ≤ 6, it is unable to predict the increased risk of relapse over the middle term. In this regard, they describe that the efficacy of the scale in preoperatively predicting an increased risk of recurrence after Bankart repair is limited in low-risk populations(93).
In a recent publication, Di Giacomo et al.(94) have reported promising results with their modification of the ISIS, incorporating the concept of GT, and which they call the Glenoid Track Instability Management Score (GTIMS). According to these authors, this modification of the scale, by using advanced imaging studies and the on-track/off-track principle, is able to more conservatively delimit the treatment of anterior instability, with promising postoperative outcomes. In general, they report minimum differences in outcomes between the two scales in addressing glenoid cavity bone defects, though they describe superior results in patients subjected to arthroscopic Bankart repair according to the GTIMS.
Surgical management scheme (Table 2)
As commented above, the percentage defect considered to be significant for surgical management decision making in patients with glenohumeral bone defects is the subject of debate; nevertheless, a summary is provided below of the data widely accepted in the literature regarding the management of these lesions.
Isolated arthroscopic soft tissue repair (Bankart repair) is indicated in the presence of an on-track non-engaging Hill-Sachs lesion with a glenoid bone loss of less than 20-25%.
Patients with a glenoid cavity defect of <25% and a Hill-Sachs lesion of >30% (or off-track) can be treated through soft tissue repair (Bankart repair), adding the remplissage technique.
In patients with an anteroinferior glenoid bone loss of 25% or more and an on-track Hill-Sachs lesion, it is advisable to use a procedure with bone grafting to augment the glenohumeral joint arch, such as the Latarjet procedure(10,12,18,95,96,97).
In patients with an anteroinferior glenoid bone loss of 25% or more and an engaging or off-track Hill-Sachs lesion, it is advisable to use the bone block protocol together with some technique to address the humeral defect, such as remplissage or bone grafts, depending on the size and location of the defect(12,13,98,99,100,101).
Conclusions
Bone defects are frequently seen in patients with anterior instability. Physical examination and imaging studies such as radiographs, CT and MRI, particularly CT with 3D reconstruction, can offer an objective preoperative analysis of the location and degree of the defect, and this added to diagnostic arthroscopy plays an important role in deciding treatment. The percentage value defining a significant glenoid cavity defect is subject to debate, and although it was initially accepted that defects of over 20-25% may be related to exclusive soft tissue treatment failure, more recently there have been reports of unfavourable outcomes with smaller percentage glenoid bone defects. Further studies are therefore needed to determine these limits and establish consensus. Hill-Sachs lesions are to be evaluated jointly with the glenoid lesion, and in this respect GT is a useful tool for assessing engagement of these bipolar lesions. The Latarjet procedure is widely used in application to glenoid bone defects, in addition to other bone block procedures described in the literature. Thus, and since they afford increased shoulder stability compared with the normal shoulder, they may be a good choice for the treatment of contact sports athletes. In the case of engaging humeral bone defects or lesions combined with glenoid defects, remplissage is a widely used procedure which in reference to external rotation may exert an influence upon the postoperative range of motion.
Figuras
Figure 1. Evaluation of instability with bone defect. Schematic representation of measurement of the real diameter of the contralateral glenoid cavity (D) and of the glenoid bone defect (d) for calculating glenoid track (GT) based on the following formula: 0.83 × (D − d).
Figure 3. Measurement of the Hill-Sachs interval (HSI): this is the result of measuring the Hill-Sachs width (HS) plus the bone bridge (BB) between the medial margin of the insertion of the cuff and the lateral region of the Hill-Sachs lesion: HSI = HS + BB. RC: insertion of the rotator cuff.
Figure 4. View of the glenoid cavity from the anterosuperior port. Arthroscopic measurement of the glenoid bone defect. Posterior radius: A (mm). Diameter of the glenoid cavity = 2A. Anterior measurement: B (mm). Defect: A − B = d mm (bone loss). Percentage defect: d / 2A = D%.
Figure 5. Latarjet procedure, arthroscopic technique. View of arthroscopic coracoid (CO) fixation from the J portal. C: guide cannula for placing the screws; G: anteroinferior part of the glenoid cavity.
Tablas
Información del artículo
Cita bibliográfica
Autores
León Ezagüi
Hospital El Pilar. Grupo Quirónsalud. Barcelona
Luca Baggio
Unidad de Hombro y Codo. Servicio de Cirugía Ortopédica y Traumatología. MC Mutual. Barcelona
María Brotat Rodríguez
Unidad de Hombro y Codo. Hospital Universitario Infanta Elena. Valdemoro, Madrid
Servicio de Cirugía Ortopédica y Traumatología. HU Infanta Elena. Valdemoro. Madrid
Abel Gómez Cáceres
Instituto Malagueño de Traumatología Deportiva (IMATDE). Málaga
José Carlos Yebra Pareja
Servicio de Cirugia Ortopedica y Traumatología. Hospital Marqués Valdecilla. Santander
Servicio de Cirugia Ortopedica y Traumatología. Hospital Clínica Mompía. Santander
Servicio de Traumatología y Cirugía Ortopédica. Complejo Hospitalario Torrecárdenas. Almería
E. P. Hospital de Poniente. El Ejido, Almería
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved experiments in humans or in animals.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgements
Thanks are due to the reviewers participating in this article and to Drs. Miguel García-Navlet and Abdul Ilah-Hachem for their effort and guidance.
Young Arthroscopy Group (Grupo Joven de Artroscopia [GJA])
This study has been coordinated and carried out within the context of the Young Arthroscopy Group (Grupo Joven de Artroscopia [GJA]) project of the Spanish Association of Arthroscopy (Asociación Española de Artroscopia [AEA]).
Referencias bibliográficas
-
1Piasecki DP, Verma NN, Romeo AA, et al. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17:482-93.
-
2Chen AL, Hunt SA, Hawkins RJ, et al. Management of bone loss associated with recurrent anterior glenohumeral instability. Am J Sports Med. 2005;33:912-25.
-
3Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60:1-16.
-
4Urayama M, Itoi E, Sashi R, et al. Capsular elongation in shoulders with recurrent anterior dislocation. Quantitative assessment with magnetic resonance arthrography. Am J Sports Med. 2003;31:64-7.
-
5Wang RY, Arciero RA. Treating the athlete with anterior shoulder instability. Clin Sports Med. 2008;27:631-48.
-
6Robinson CM, Howes J, Murdoch H, et al. Functional outcome and risk of recurrent instability after primary traumatic anterior shoulder dislocation in young patients. J Bone Joint Surg Am. 2006;88:2326-36.
-
7Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82:35-46.
-
8Lynch JR, Clinton JM, Dewing CB, et al. Treatment of osseous defects associated with anterior shoulder instability. J Shoulder Elbow Surg. 2009;18:317-28.
-
9Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy. 2007;23:1033-41.
-
10Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677-94.
-
11Yamamoto N, Muraki T, Sperling JW, et al. Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Joint Surg. 2010;92:2059-66.
-
12Di Giacomo G, Itoi E, Burkhart S. Evolving Concept of Bipolar Bone Loss and the Hill-Sachs Lesion: From “Engaging/Non-Engaging” Lesion to “On-Track/Off-Track” Lesion. Arthroscopy. 2014;30(1):90-8.
-
13Ochoa E Jr, Burkhart SS. Glenohumeral bone defects in the treatment of anterior shoulder instability. Instr Course Lect. 2009;58:323-36.
-
14Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am J Sports Med. 1997;25:306-11.
-
15Calandra JJ, Baker CL, Uribe J. The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations. Arthroscopy. 1989;5:254-7.
-
16Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A:878-84.
-
17Griffith JF, Antonio GE, Yung PS, et al. Prevalence, pattern, and spectrum of glenoid bone loss in anterior shoulder dislocation: CT analysis of 218 patients. AJR Am J Roentgenol. 2008;190:1247-54.
-
18Boileau P, Villalba M, Hery JY, et al. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88:1755-63.
-
19Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20:169-74.
-
20Burkhart SS, Danaceau SM. Articular arc length mismatch as a cause of failed Bankart repair. Arthroscopy. 2000;16:740-4.
-
21Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res. 2002;400:65-76.
-
22Yamamoto N, Itoi E, Abe H, Kikuchi K. Effect of an Anterior Glenoid Defect on Anterior Shoulder Stability: A Cadaveric Study. Am J Sports Med. 2009;37(5):949-54.
-
23Montgomery WH Jr, Wahl M, Hettrich C, et al. Anteroinferior bone-grafting can restore stability in osseous glenoid defects. J Bone Joint Surg Am. 2005;87(9):1972-7.
-
24Widjaja AB, Tran A, Bailey M, Proper S. Correlation between Bankart and Hill-Sachs lesions in anterior shoulder dislocation. ANZ J Surg. 2006;76:436-8.
-
25Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-9.
-
26Gottschalk LJ 4th, Walia P, Patel RM, et al. Stability of the Glenohumeral Joint With Combined Humeral Head and Glenoid Defects: A Cadaveric Study. Am J Sports Med. 2016;44(4):933-40.
-
27Shaha JS, Cook JB, Song DJ, et al. Redefining "Critical" Bone Loss in Shoulder Instability: Functional Outcomes Worsen With "Subcritical" Bone Loss. Am J Sports Med. 2015;43(7):1719-25.
-
28Hintermann B, Gächter A. Arthroscopic findings after shoulder dislocation. Am J Sports Med. 1995;23(5):545-51.
-
29Cho SH, Cho NS, Rhee YG. Preoperative analysis of the Hill-Sachs lesion in anterior shoulder instability: how to predict engagement of the lesion. Am J Sports Med. 2011;39(11):2389-95.
-
30Di Giacomo G, Golijanin P, Sánchez G, Provencher M. Radiographic analysis of the Hill-Sachs lesion in anteroinferior shoulder instability after first-time dislocations. Arthoscopy. 2016;32:1509-14.
-
31Kawakami J, Yamamoto N, Hatta T, Shinagawa K, Itoi E. In Which Arm Position Is a Hill-Sachs Lesion Created? Am J Sports Med. 2019;47(10):2464-8.
-
32Yamamoto N, Itoi E, Abe H, Kikuchi K. Effect of an Anterior Glenoid Defect on Anterior Shoulder Stability: A Cadaveric Study. Am J Sports Med. 2009;37(5):949-54.
-
33Itoi E, Yamamoto N, Hatta T, Kawakami J. Overview of Evaluation and Management of the Unstable Shoulder With and Without Bone Loss: Definition, Measurement, and Guidelines on Treatment. In: Imhoff A, Savoie III F (eds.). Shoulder Instability Across the Life Span. Berlin: Springer, Heidelberg; 2017.
-
34Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16:649-56.
-
35Itoi E, Lee SB, Amrami KK, et al. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med. 2003;31:112-8.
-
36Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. Am J Roentgenol. 2003;180:1423-30.
-
37Bushnell BD, Creighton RA, Herring MM. Bony instability of the shoulder. Arthroscopy. 2008;24:1061-73.
-
38Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24:376-82.
-
39Shi L, Griffith JF, Huang J, Wang D. Excellent side-to-side symmetry in glenoid size and shape. Skeletal Radiol. 2013;42:1711-5.
-
40Griffith JF, Yung PS, Antonio GE, Tsang PH, Ahuja AT, Chan KM. CT compared with arthroscopy in quantifying glenoid bone loss. AJR Am J Roentgenol. 2007;189:1490-3.
-
41Griffith J. Measuring glenoid and humeral bone loss in shoulder dislocation. Quant Imaging Med Surg. 2019;9(2):134-43.
-
42Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85(5):878-84.
-
43Lansdown DA, Wang K, Bernardoni E, Frank RM, Yanke AB, Cole BJ, et al. Variability in the Contour of Cadaveric Anterior and Posterior Glenoids Based on Ipsilateral 3-Dimensional Computed Tomography Reconstructions: Implications for Clinical Estimation of Bone Loss. Arthroscopy. 2018;34(9):2560-6.
-
44Gyftopoulos S, Hasan S, Bencardino J, et al. Diagnostic accuracy of MRI in the measurement of glenoid bone loss. AJR Am J Roentgenol. 2012;199(4):873-8.
-
45Maio M, Sarmento M, Moira N, Cartucho A. How to measure a Hill–Sachs lesion: a systematic review. EFORT Open Rev. 2019;4(4):151-7.
-
46Stillwater L, Koenig J, Maycher B, Davidson M. 3D-MR vs. 3D-CT of the shoulder in patients with glenohumeral instability. Skeletal Radiol. 2017;46(3):325-31.
-
47Gyftopoulos S, Beltran LS, Bookman J, Rokito A. MRI Evaluation of Bipolar Bone Loss Using the On-Track Off-Track Method: A Feasibility Study. AJR Am J Roentgenol. 2015;205:848-52.
-
48Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16:649-56.
-
49Huijsmans PE, Haen PS, Kidd M, Dhert WJ, van der Hulst VP, Willems WJ J. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: a cadaveric study. Shoulder Elbow Surg. 2007;16(6):803-9.
-
50Schneider AK, Hoy GA, Ek ET, et al. Interobserver and intraobserver variability of glenoid track measurements. J Shoulder Elbow Surg. 2017;26(4):573-9.
-
51Momaya AM, Tokish JM. Applying the Glenoid Track Concept in the Management of Patients with Anterior Shoulder Instability. Curr Rev Musculoskelet Med. 2017;10(4):463-8.
-
52Yamamoto N, Shinagawa K, Hatta T, Itoi E. Peripheral-Track and Central-Track Hill-Sachs Lesions: A New Concept of Assessing an On-Track Lesion. Am J Sports Med. 2020;48(1):33-8.
-
53Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18(5):488-91.
-
54Helfet AJ. Coracoid transplantation for recurring dislocation of the shoulder. J Bone Joint Surg Br. 1958;40:198-202.
-
55Mead NC, Sweeney HJ. Bristow procedure (letter). Spectator. 1964 July 9.
-
56Barth J, Boutsiadis A, Neyton L, Lafosse L, Walch G. Can a Drill Guide Improve the Coracoid Graft Placement During the Latarjet Procedure? A Prospective Comparative Study With the Freehand Technique. Orthop J. Sports Med. 2017;5(10):232596711773421.
-
57Lafosse L, Boyle S. Arthroscopic Latarjet procedure. J Shoulder Elbow Surg. 2010;19:2-12.
-
58Boileau P, Mercier N, Roussanne Y, Thelu CE, Old J. Arthroscopic Bankart-Bristow-Latarjet procedure: the development and early results of a safe and reproducible technique. Arthroscopy. 2010;11:1434-50.
-
59Latarjet M. A propos du traitement des luxations récidivants de l’épaule. Lyon Chir. 1954;49:994-1003.
-
60May VR. A modified Bristow operation for anterior recurrent dislocation of the shoulder. J Bone Joint Surg Am. 1970;52:1010-6.
-
61Patte D, Bernageau J, Bancel P. The anteroinferior vulnerable point of the glenoid rim. In: Bateman, Welsch (ed.). Surgery of the shoulder. New York: Marcel Dekker; 1985. pp. 94-9.
-
62De Beer JF, Roberts C. Glenoid bone defects–open latarjet with congruent arc modification. Orthop Clin North Am. 2010;41(3):407-15.
-
63Allain J, Goutallier D, Glorion C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am. 1998;80:841-52.
-
64Hovelius L, Sandström B, Olofsson A, Svensson O, Rahme H. The effect of capsular repair, bone block healing, and position on the results of the Bristow-Latarjet procedure (study III): long-term follow-up in 319 shoulders. J Should Elbow Surg. 2012;21(5):647-60.
-
65Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202.
-
66Neyton L, Young A, Dawidziak B, et al. Surgical treatment of anterior instability in rugby union players: clinical and radiographic results of the Latarjet-Patte procedure with minimum 5-year follow-up. J Shoulder Elbow Surg. 2012;21(12):1721-7.
-
67Hart R, Sváb P, Krejzla J. Modified Latarjet procedure for recurrent shoulder dislocation in elderly patients. Acta Chir Orthop Traumatol Cech. 2010;77(2):105-11.
-
68Schmid SL, Farshad M, Catanzaro S, Gerber C. The Latarjet procedure for the treatment of recurrence of anterior instability of the shoulder after operative repair: a retrospective case series of forty-nine consecutive patients. J Bone Joint Surg. 2012;94(11):e75.
-
69Boileau P, Mercier N, Roussanne Y, Thélu CÉ, Old J. Arthroscopic Bankart-Bristow-Latarjet procedure: the development and early results of a safe and reproducible technique. Arthroscopy. 2010;26(11):1434-50.
-
70Warner JJ, Gill TJ, O’Hollerhan JD, et al. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med. 2006;34:205-12.
-
71Gebhard F, Dragees M, Steinmann R, Hoellen I, Hartel W. Functional outcome of Eden-Hybinette-Lange operation in post-traumatic recurrent shoulder dislocation. Der Unfallchirurg. 1997;100:770-5.
-
72Auffarth A, Schauer J, Matis N, et al. The J-bone graft for anatomical glenoid reconstruction in recurrent posttraumatic anterior shoulder dislocation. Am J Sports Med. 2008;36:638-47.
-
73Provencher MT, Frank RM, Golijanin P, et al. Distal Tibia Allograft Glenoid Reconstruction in Recurrent Anterior Shoulder Instability: Clinical and Radiographic Outcomes. Arthroscopy. 2017;33(5):891-7.
-
74Taverna E, D'Ambrosi R, Perfetti C, Garavaglia G. Arthroscopic bone graft procedure for anterior inferior glenohumeral instability. Arthrosc Tech. 2014;3:e653-e660.
-
75Boileau P, Duysens C, Saliken D, Lemmex DB, Bonnevialle N. All-arthroscopic, guided Eden-Hybbinette procedure using suture-button fixation for revision of failed Latarjet. J Shoulder Elbow Surg. 2019;28:e377-e388.
-
76Hachem AI, Del Carmen M, Verdalet I, Rius J. Arthroscopic Bone Block Cerclage: A Fixation Method for Glenoid Bone Loss Reconstruction Without Metal Implants. Arthrosc Tech. 2019;8(12):e1591-e1597.
-
77Zhao J, Huangfu X, Yang X, Xie G, Xu C. Arthroscopic glenoid bone grafting with nonrigid fixation for anterior shoulder instability: 52 patients with 2- to 5-year follow-up. Am J Sports Med. 2014;42:831-9.
-
78Whelan A, Coady C, Ho-Bun Wong I. Anterior glenohumeral capsular reconstruction using a human acellular dermal allograft. Arthrosc Tech. 2018;7:e1235-e1241.
-
79Regan Jr WD, Webster-Bogaert S, Hawkins RJ, Fowler PJ. Comparative functional analysis of the Bristow, Magnuson-Stack, and Putti-Platt procedures for recurrent dislocation of the shoulder. Am J Sports Med. 1989;17:42-8.
-
80Weber BG, Simpson LA, Hardegger F. Rotational humeral osteotomy for recurrent anterior dislocation of the shoulder associated with a large Hill-Sachs lesion. J Bone Joint Surg. 1984;66:1443-50.
-
81Saltzman BM, Riboh JC, Cole BJ, Yanke AB. Humeral Head Reconstruction With Osteochondral Allograft Transplantation. Arthroscopy. 2015;31(9):1827-34.
-
82Wolf EM, Pollack M, Smalley C. Hill-Sachs "Remplissage": an arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy. 2007;23:e1-2.
-
83Kazel MD, Sekiya JK, Greene JA, Bruker CT. Percutaneous correction (humeroplasty) of humeral head defects (Hill-Sachs) associated with anterior shoulder instability: a cadaveric study. Arthroscopy. 2005;12:1473-8.
-
84Re P, Gallo RA, Richmond JC. Transhumeral head plasty for large Hill-Sachs lesions. Arthroscopy. 2006;22:e1-4.
-
85Connolly JF. Humeral head defects associated with shoulder dislocation: their diagnostic and surgical significance. Instr Course Lect. 1972;21:42-54.
-
86Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-Sachs "remplissage": an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24:723-6.
-
87Koo SS, Burkhart SS, Ochoa E. Arthroscopic double-pulley remplissage technique for engaging Hill-Sachs lesions in anterior shoulder instability repairs. Arthroscopy. 2009;25(11):1343-8.
-
88Nourissat G, Kilinc AS, Werther JR, Doursounian L. A prospective, comparative, radiological, and clinical study of the influence of the "remplissage" procedure on shoulder range of motion after stabilization by arthroscopic Bankart repair. Am J Sport Med. 2011;39(10):2147-52.
-
89Franceschi F, Papalia R, Rizzello G, et al. Remplissage repair-new frontiers in the prevention of recurrent shoulder instability: a 2-year follow-up comparative study. Am J Sports Med. 2012;40(11):2462-9.
-
90Elkinson I, Giles JW, Faber KJ, et al. The effect of the remplissage procedure on shoulder stability and range of motion: an in vitro biomechanical assessment. J Bone Joint Surg. 2012;94(11):1003-12.
-
91Boileau P, O'Shea K, Vargas P, et al. Anatomical and functional results after arthroscopic Hill-Sachs remplissage. J Bone Joint Surg. 2012;94(7):618-26.
-
92Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-7.
-
93Ruiz Ibán MA, Asenjo Gismero CV, Moros Marco S, et al. Instability severity index score values below 7 do not predict recurrence after arthroscopic Bankart repair. Knee Surg Sports Traumatol Arthrosc. 2019 Dec;27(12):3905-11.
-
94Di Giacomo G, Peebles LA, Pugliese M, et al. Glenoid Track Instability Management Score: Radiographic Modification of the Instability Severity Index Score. Arthroscopy. 2020;36(1):56-67.
-
95Yamamoto N, Itoi E, Abe H, et al. Effect of an anterior glenoid defect on anterior shoulder stability: a cadaveric study. Am J Sports Med. 2009;37:949-54.
-
96Abrams JS. Role of arthroscopy in treating anterior instability of the athlete’s shoulder. Sports Med Arthrosc. 2007;15:230-8.
-
97Plath JE, Henderson DJH, Coquay J, Dück K, Haeni D, Lafosse L. Does the arthroscopic Latarjet procedure effectively correct "off-track" Hill-Sachs lesions? Am J Sports Med. 2017:363546517728717.
-
98Miniaci A, Berlet G, Hand C, et al. Segmental humeral head allografts for recurrent anterior instability of the shoulder with large Hill-Sachs defects: a two to 8 year follow up. J Bone Joint Surg Br. 2008;90(suppl I):86.
-
99Purchase RJ, Wolf EM, Hob-Good ER, et al. Hill-Sachs ‘‘remplissage’’: an arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy. 2008;24:723-6.
-
100Toro F, Melean P, Moraga C, et al. Remplissage: infraspinatus tenodesis and posterior capsulodesis for the treatment of Hill Sachs lesions: an all intraarticular technique. Tech Shoulder Elbow Surg. 2008;9:188-92.
-
101Bushnell BD, Creighton RA, Herring MM. Hybrid treatment of engaging Hill-Sachs lesions: arthroscopic capsulolabral repair and limited posterior approach for bone grafting. Tech Shoulder Elbow Surg. 2007;8:194-203.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Don’t forget to remember
- Anatomical study on subtalar arthrodesis: anterolateral versus posterior portals
- Customized 3D printed guides for the repair of rupture of the posterior root of the internal meniscus. Preliminary outcomes
- Anatomical ligament reconstruction with allograft in chronic acromioclavicular dislocations restores joint stability and function. Short-term outcomes in 14 cases
- Patellar tendinopathy: diagnostic approach and functional assessment scales
- Current management of posterior cruciate ligament rupture. A narrative review
- Management of anterior instability of the shoulder with bone defects
- Open anatomical reconstruction surgical technique with allograft for the treatment of chronic acromioclavicular dislocations
- Simultaneous but different damage to both posterior meniscus roots with multiple ligament injury of the knee following high-energy trauma. Presentation of a case
- Pigmented villonodular synovitis of the ankle
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.