Autologous chondrocyte implant surgery of the knee
Cirugía de implante de condrocitos autólogos en rodilla
Resumen:
El cartílago es un tejido que no se regenera y cuya lesión provoca incapacidad y degeneración de la articulación. De esta manera, cuando este se lesiona, da lugar a la artrosis, con lo que supone de dolor para el paciente y limitación funcional. La técnica del cultivo celular es la única esperanza de conseguir regenerar los tejidos lesionados. El implante de condrocitos es una técnica que consigue regenerar el cartílago articular, consiguiendo disminuir el dolor o incluso hacerlo desaparecer, y además alcanzar la funcionalidad necesaria para las articulaciones.
La técnica consiste en tomar una biopsia de cartílago de la articulación dañada mediante una cirugía artroscópica. Esta muestra se manda al laboratorio de cultivo celular, durante 4-6 semanas cultivan los condrocitos y los multiplican hasta alcanzar 5.000.000 de condrocitos por centímetro cuadrado. Cuando el cultivo está listo, se realiza una segunda cirugía para el implante. En esta cirugía, tras el abordaje de la rodilla y la exposición de la lesión, se limpia bien la misma, dejando bordes de la lesión con cartílago sano. Sembramos las células en la membrana de colágeno, en su lado rugoso, debiendo esperar unos 14 minutos con el fin de que las células queden bien adheridas a la membrana. Colocamos la membrana con células en la lesión con el lado rugoso hacia el hueso subcondral y procedemos a la sutura de la membrana al cartílago sano adyacente. Se puede realizar esta sutura con Vicryl® de 5/0 oscuro o con el instrumental específico para sutura ósea con Vicryl®. Finalmente, se sellan los bordes con Tissucol® (fibrina). Tras esperar unos 5 minutos, se comprueba la estabilidad de la sutura, realizando varios ciclos de flexoextensión de la rodilla. Se cierra la articulación con sutura por planos.
Se considera al implante de condrocitos autólogos como la mejor técnica para conseguir regenerar el cartílago con terapia celular, consiguiendo así una verdadera medicina regenerativa. Presenta, además, una oportunidad cuando el resto de los tratamientos ha fracasado. Asimismo, es una técnica segura con pocas complicaciones.
Abstract:
Cartilage is a tissue that fails to regenerate, and damage to it results in disability and degeneration of the affected joint. Damage to a joint gives rise to osteoarthrosis, with pain and functional limitation. The application of cell culture technology is the only hope for regeneration of the damaged tissues. Chondrocyte implantation is able to regenerate joint cartilage, with a decrease or even elimination of the pain, and moreover affords necessary joint function.
The technique involves the obtainment of a biopsy of the damaged joint through arthroscopic surgery. The sample is then sent to the cell culture laboratory, where the chondrocytes are cultured for 4-6 weeks and multiplied until reaching 5 million chondrocytes per square centimetre. When the culture is ready, second surgery for implantation is carried out. In this operation, after accessing the knee and exposing the lesion, the latter is carefully cleaned, leaving lesion margins with healthy cartilage. The cells are seeded on the rough side of a collagen membrane, and we need to wait about 14 minutes in order for the cells to become well adhered to the membrane. The membrane with the cells is placed in the lesion with the rough side facing the subchondral bone, and is sutured to the healthy adjacent cartilage. Suturing can be done using dark Vicryl® 5/0 or with the specific instruments for bone suture with Vicryl®. The margins are finally sealed with Tissucol® (fibrin glue). After waiting approximately 5 minutes, the stability of the suture is checked, performing several flexion-extension cycles of the knee. Layered suturing is performed to close the joint.
Autologous chondrocyte implantation is considered to be the best technique for joint regeneration with cell therapy, constituting genuine regenerative medicine. This strategy moreover represents an opportunity when the rest of treatments have failed. On the other hand, the technique is safe and has few complications.
Introduction
Many surgical techniques are used in an attempt to repair damaged joint cartilage(1). The most common procedures are bone marrow stimulation techniques that involve bleeding of the subchondral bone to produce lesion repair. These techniques have been widely used in their different versions: perforations, microfractures or nanofractures - but the results are short-lasting, and good outcomes are only achieved in the case of small lesions. As an alternative to such procedures, different restoration techniques have been developed which - as their name indicates - seek to restore the structure and thus also the function of the affected tissue. These techniques comprise mosaicplasty, osteochondral grafts and cell therapy methods.
This surgery video presents a cell therapy technique that is able to regenerate the damaged tissue (the cartilage), and moreover constitutes an autologous procedure, i.e., it produces no patient immune response(2).
Indications
- Outerbridge grade III-IV lesions(3).
- Injuries of the hip, knee and ankle.
- Patients between 18-55 years of age.
- Rescue treatment in cases of failed techniques.
Contraindications
- Active infection.
- Tumours.
- Associated diseases: these should be treated first.
- Patients over 55 or under 18 years of age.
Surgical technique
Autologous chondrocyte implantation technique is performed in two surgical steps (Table 1).
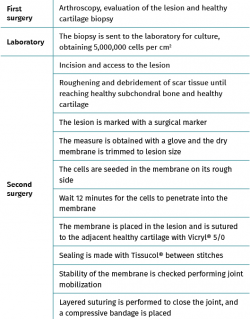
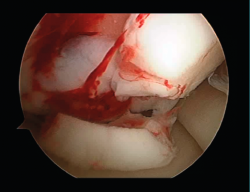
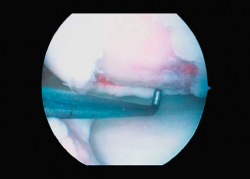
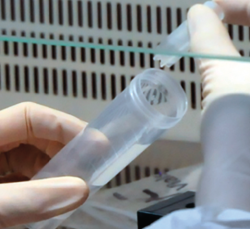
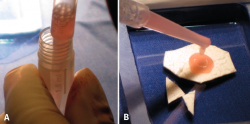
First surgery involves arthroscopy of the damaged joint. It assesses the chondral lesion to be treated and confirms whether it is amenable to the chondrocyte implantation technique (Figures 1 and 2). Other procedures are also carried out if needed, such as for example reconstruction of the anterior cruciate ligament, partial meniscectomies, meniscus implantation, patellar realignments, osteotomies, etc. Lastly, the cartilage sample is taken from a non-weight bearing zone (intercondylar zone or internal femoral condyle at its upper margin). The sample is extracted with biopsy forceps or, alternatively, using discectomy forceps. Between 3-4 rice grain-sized fragments of healthy cartilage are harvested (Figure 3). The biopsy material is placed in a sterile receptacle containing a culture medium (DMEN) (Figure 4). The material is kept at room temperature and is shipped to the laboratory as quickly as possible. A form should be completed (Figure 5), stating the joint, the location of the lesion and the size of the defect. Once in the laboratory, the sample is processed and cultured. After 4-6 weeks (depending on the case), the culture is ready for implantation.
Second surgery involves implantation of the chondrocytes. This is done by open surgery, though in some cases an arthroscopic approach can be used if allowed by the size or location of the lesion. The procedure involves the following steps:
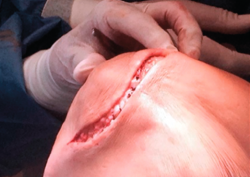
- A paramedial joint incision is made, with luxation of the knee and access to the lesion (Figure 6).
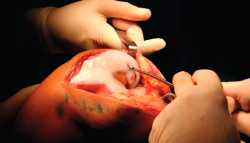
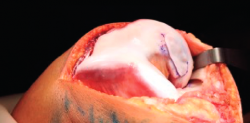
- The damaged cartilage is cleaned using curettes, with debridement of the defect, and the lesion is left with healthy and exposed subchondral bone (Figure 7).
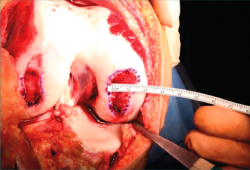
- A surgical marker is used to mark the margins of the lesion, and a mould of the lesion is obtained with a piece of glove (Figure 8).
- Patellar reduction is then performed, a humid dressing is placed in the joint, and cell implantation in the membrane is carried out.
- The glove is cut along the mark on the instrument table, checking again that the measurement is correct. The dry membrane is trimmed to the size of the lesion. The membrane is placed with the rough zone facing upwards (Figure 9). The cells are received from the laboratory in a sterile jar. Using a sterile pipette, all the cells are placed on the membrane, distributing them well over the entire surface(4) (Figure 10).
- We have to wait 12 minutes for the cells to penetrate into the membrane (the specific time will be indicated by the membrane laboratory).
- We then return to the joint, wash it, and carefully dry the lesion bed to ensure the absence of bleeding.
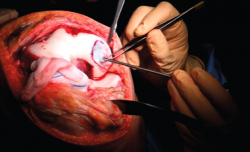
- The membrane is placed in the lesion and the margins of the former are sutured to the healthy cartilage with Vicryl® 5/0 (Figures 11 and 12).
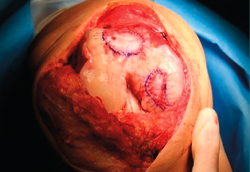
- We check that the membrane is well fixed and seal between the stitches with Tissucol® (Figure 13). A waiting period of 3-5 minutes is observed, and the joint is mobilized before closure to check that the membrane does not detach with movement of the joint.
- Layered suturing is performed to close the joint, and a compressive bandage is placed.
Postoperative period
The patient must spend two months without weight bearing, though the knee can be mobilized to preserve mobility and a good joint range. Gradual weight bearing starts after two months, with crutches. After 2-4 months, physiotherapy starts to restore normal joint range and support the extremity. Exercises against resistance, together with static bicycling and swimming, begin after four months.
Limb strengthening with weights can begin after 6 months. Jump and subsequently running training starts after 10 months, and contact sports can begin after 12 months.
Results
All our patients undergo controls and study. We use the International Knee Documentation Committee (IKDC) questionnaire before surgery, after 6 months, and then every year(5). In addition, magnetic resonance imaging (MRI) controls are made at 3, 6 and 9 months, and one year. Annual controls continue thereafter. On the other hand, demographic and clinical data are compiled on occasion of the periodic review visits(6).
All the results are studied and entered in our database to allow follow-up and assessment of the technique. Our results are also compared against those of other authors(7).
The results of our group were published in Cartilage in 2018(8). They correspond to the first 50 patients treated with the autologous cartilage implantation of the knee technique and with a minimum follow-up period of two years. The mentioned publication evidences improvement of the visual analogue scale (VAS) and IKDC score one and two years after surgery, with statistically significant differences.
Possible complications
In our case series there were no implant detachment or delamination problems, though such situations have been reported by other authors. We have recorded cases of arthrofibrosis, with difficulty gaining mobility, that were resolved through arthroscopic arthrolysis. We also have had cases that have not shown clinical improvement.
Conclusions
Autologous chondrocyte implantation (Instant CEMTRO Cell [ICC]) is effective for the treatment of knee cartilage defects measuring over 1.5 cm2 in size, affording significant reduction of pain, effusion and crepitus, and increasing mobility over follow-up.
According to subjective patient perception as assessed with the IKDC questionnaire, both knee symptoms and function improve significantly with high-density chondrocyte implants at one and two years after implantation.
The MRI studies evidence correct implant integration after two years. These imaging studies also show a reduced number of bone edemas versus the reports of other investigators, particularly in comparison with the MACI (matrix-assisted autologous chondrocyte implantation) technique.
The small percentage of patients with complications recorded after follow-up shows that autologous chondrocyte implantation (ICC) is safe for the treatment of chondral lesions and may be regarded as a rescue option when other techniques have failed.
Annexed material. Surgery video: chondrocyte implantation
Tablas
Figuras
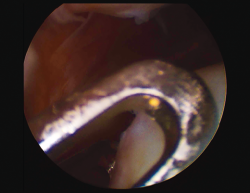
Figure 1. Arthroscopic view of an Outerbridge grade IV acute chondral lesion of the internal femoral condyle.
Figure 2. Arthroscopic view of an Outerbridge grade IV chronic chondral lesion of the internal femoral condyle.
Figure 3. View showing biopsy of the cartilage of the internal femoral condyle, internal and upper margin.
Figure 10. A: the cells are collected with the pipette; and B: the cells are distributed over the previously trimmed membrane.
Información del artículo
Cita bibliográfica
Autores
Isabel Guillén Vicente
Unidad de Cartílago. Clínica CEMTRO. Madrid
Unidad de Tobillo y Pie. Clínica CEMTRO. Madrid
Unidad de Rodilla. Clínica CEMTRO. Madrid
Fernando Sanz Zapata
Unidad de Rodilla. Clínica CEMTRO. Madrid
Pedro Guillén García
Unidad de pie y tobillo. Clínica CEMTRO. Madrid
Servicio de Traumatología. Clínica CEMTRO. Madrid
Unidad de Investigación Biomédica. Clínica CEMTRO. Madrid. España
Ethical responsibilities
Conflicts of interest. The authors declare that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that the procedures carried out abided with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58-69.
-
2Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:272481.
-
3Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752-7.
-
4Guillén-García P, Rodríguez-Íñigo E, Guillén-Vicente I, Caballero-Santos R, Guillén-Vicente M, Abelow S, et al. Increasing the Dose of Autologous Chondrocytes Improves Articular Cartilage Repair: Histological and Molecular Study in the Sheep Animal Model. Cartilage. 2014;5:114-22.
-
5Greco NJ, Anderson AF, Mann BJ, Cole BJ, Farr J, Nissen CW, Irrgang JJ. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38:891-902.
-
6Guillén-García P, Rodríguez-Íñigo E, Aráuz S, Guillén-Vicente M, Guillén-Vicente I, Caballero-Santos R, et al. Nuestra experiencia con la técnica de implante de condrocitos autólogos para el tratamiento de lesiones condrales: resultados de 50 pacientes a 2 años de seguimiento. Rev Esp Artrosc Cir Articul. 2015;22:120-5.
-
7Brittberg M. Autologous chondrocyte implantation-technique and long term follow-up. Injury. 2008;39:S40-S49.
-
8López-Alcorocho JM, Aboli L, Guillén-Vicente I, Rodríguez-Íñigo E, Guillén-Vicente M, Fernández-Jaén TF, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. 2018 Oct;9(4):363-9.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Update on the treatment of focal cartilage lesions of the knee
- Comparison of the intraarticular injection of platelet rich plasma (PRGF®) and hyaluronic acid (Hyalone®) in the treatment of chondral lesions: a randomized, prospective clinical study
- Microfractures or bone marrow stimulation (BMS): evolution of the technique
- Fresh osteochondral graft. Indications, surgical technique and scientific evidence
- Autologous matrix-induced chondrogenesis (AMIC)
- Instant CEMTRO Cell (ICC), high density autologous chondrocytes implantation
- Regeneration of joint cartilage: perspectives and future
- Autologous chondrocyte implant surgery of the knee
- Full thickness chondral ulcers of the patella and femoral condyle of the knee
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.