Management of anterior glenohumeral instability without significant glenoid bone damage
Manejo de la inestabilidad glenohumeral anterior sin lesión ósea glenoidea significativa
Resumen:
La inestabilidad glenohumeral es una entidad clínica relevante que cuenta con una alta incidencia y prevalencia. El objetivo de este trabajo es realizar una revisión de los principales aspectos del manejo de las inestabilidades glenohumerales traumáticas anteriores, con afectación exclusiva de partes blandas.
La anamnesis es de gran importancia; ante un primer episodio de luxación debe ir encaminada a investigar características de la luxación y si existen factores de riesgo de recidiva. La anamnesis ante la sospecha de inestabilidad tiene por objetivo ayudar a confirmar el diagnóstico y a establecer su gravedad.
La exploración física junto con las pruebas diagnósticas permiten confirmar el diagnóstico y descartar lesiones asociadas.
El tratamiento tras un primer episodio de luxación glenohumeral es conservador. Se debe considerar el tratamiento quirúrgico tras un primer episodio en aquellos casos que asocien factores de riesgo de recidiva.
El tratamiento de la inestabilidad glenohumeral recurrente es quirúrgico. El tratamiento quirúrgico básicamente se resume en técnicas de partes blandas o técnicas con aporte óseo. La decisión entre realizar unas u otras depende de la magnitud del defecto óseo, por lo que es importante definir ese límite. Clásicamente, se consideraba que un defecto glenoideo del 25% junto con los conceptos de on-track y off-track de las lesiones de Hill Sachs definen los límites para el uso de una técnica de partes blandas o de aporte óseo. Pero en la actualidad se está considerando bajar el límite de defecto óseo glenoideo; cada vez hay más autores que aceptan considerar un defecto de un 20% o incluso un 15% si asocia otros factores de riesgo de recidiva.
En nuestro trabajo describiremos las técnicas quirúrgicas de partes blandas más frecuentes para el tratamiento de inestabilidades sin lesiones óseas relevantes, es decir, que se encuentren por debajo de los límites previamente descritos.
Abstract:
Glenohumeral instability is a clinically relevant condition with a high incidence and prevalence. The present review examines the main management aspects referred to traumatic anterior glenohumeral instability with exclusive soft tissue involvement.
The case history is of great importance; in the event of a first dislocation episode, we should explore the characteristics of the lesion and the presence of risk factors for relapse. When instability is suspected, the case history seeks to help confirm the diagnosis and establish the degree of severity of the condition.
The physical examination and diagnostic tests allow us to confirm the diagnosis and discard associated lesions.
Conservative management is indicated following a first episode of glenohumeral dislocation. Surgical treatment should be considered following a first episode in those cases presenting risk factors for relapse.
Surgery is indicated in patients with recurrent glenohumeral instability. Surgical treatment is basically divided into soft tissue techniques or bone grafting techniques. The decision to use one surgical approach or the other depends on the magnitude of the bone defect; it is therefore important to define this limit. Classically, a glenoid defect of 25% together with the on-track and off-track concepts of Hill Sachs lesions were considered to define the limits for the use of a soft tissue technique or bone grafting technique. Lowering of the limit of the glenoid bone defect is currently being considered, however, an increasing number of authors accept a defect of 20% or even 15% if associated to other risk factors for relapse.
The present study describes the most frequent soft tissue surgical techniques for treating instabilities without relevant bone lesions, i.e., those below the previously described limits.
Introduction
Glenohumeral instability is defined as the symptomatic incapacity to keep the humeral head centred within the glenoid fossa. It can give rise to pain, functional impairment and/or subjective anxiety(1).
The term instability encompasses a broad range of pathological conditions: from cases limited to apprehension (concern that the shoulder may dislocate or suffer subluxation in certain positions) or episodes of subluxation (symptomatic partial loss of joint congruence that undergoes spontaneous reduction) to patients who have suffered two or more episodes of manifest glenohumeral dislocation (complete loss of joint congruence, requiring manual reduction)(1). On the other hand, it is important to take into account that some instabilities manifest simply in the form of pain, discomfort and/or joint failure that impede work or sports activities. These forms of clinically less expressive instability are the presentations that may go unnoticed. In these cases we may find indirect signs or lesions attributable to the instability itself and which should not be confused with a primary disease process, e.g., partial-thickness tendon injuries such as PASTA (partial articular-side supraspinatus avulsion), chondral lesions, impingement, posterointernal impairment of the rotator cuff, etc.
These are clinically relevant conditions with a high incidence of approximately 23.9/100,000 individuals a year(2), and mainly affect young and active subjects.
There are multiple causes of instability, from congenital (glenoid dysplasia, ligament hyperlaxity in systemic disorders such as Ehlers-Danlos syndrome, etc.) to acquired causes that may be of a traumatic nature, with damage to the stabilizer elements, or of an atraumatic nature, with progressive atraumatic decompensation of the stabilizer mechanisms(3).
The present review examines the main management aspects referred to traumatic anterior glenohumeral instability with exclusive soft tissue histopathological involvement, i.e., those presentations without relevant associated glenoid bone loss. The study focuses on the principal clinical and radiological aspects, underscoring those that must be taken into account in order to decide the most appropriate treatment in each case.
Case history
The case history (anamnesis) is essential for establishing the diagnosis and estimating the severity of instability. In addition, it allows us to identify possible risk factors for relapse after a first luxation episode, as well as factors indicative of a poor prognosis after treatment of recurrent instability.
A distinction must be made between anamnesis performed after a first dislocation episode and anamnesis in cases of suspected instability.
Case history following a first episode of glenohumeral dislocation
Following a first episode of glenohumeral dislocation, compilation of the case history should seek to obtain the information needed to identify risk factors for relapse and the type of luxation involved, such as:
- Patient age.
- Male or female gender.
- Dominance (whether the affected arm is the dominant extremity or not).
- Type of physical activities and sports practiced by the patient. In the case of contact sports such as rugby, we should record whether the activity is at professional or amateur level, whether movements above the head are involved, such as in volleyball or water polo, etc.
- Mechanism of injury: traumatic or non-traumatic.
- Intensity of the mechanism of injury.
- Direction of the luxation.
- Ease or difficulty of reducing the first dislocation episode.
- Associated lesions following luxation, such as distal coldness of the hand, temporary or permanent sensory alterations of the extremity, etc.
- History of instability and/or hyperlaxity of other joints.
- Related disease antecedents such as congenital or systemic disorders, or previous surgeries.
It is important to mention some of these risk factors. A meta-analysis conducted in 2015 on the risk factors that most predispose to recurrence after a first glenohumeral dislocation episode concluded that the estimated real relapse rate after a first episode is 39%, and that the most important risk factors are patient age and gender, the mechanism of the injury, the practice of contact or other sports, the presence of glenoid bone damage, hyperlaxity, and involvement of the axillary plexus(4).
An early patient age at the time of the first episode is one of the most important risk factors for relapse. In this respect, the main hypothesis is that young individuals are characterized by a predominance of tendon and ligament connective tissue elasticity, due to the specific composition of their collagen fibers, and these properties moreover modify with advancing age(5). A number of authors have reported that if the first luxation episode takes place before 20 years of age, the recurrence rate may be as high as 90%, and recurrence is more frequent in the first two years after luxation(6). However, in individuals over age 40, the recurrence rate drops to 10-15%(7). Hovelius et al. published a prospective study in which a somewhat lesser relapse rate was observed for each age group: 33% in patients under 20 years of age; 25% in those between 20-30 years of age; and 10% in individuals between 30-40 years of age. Most relapses occurred within the first two years(8).
The presence of bone lesions is also regarded as one of the most relevant risk factors(9). These lesions are present in a considerable percentage of cases after only a single luxation episode, and directly influence the recurrence rate. Grifith et al. reported the presence of anterior glenoid bone lesions in 40% of all cases after a first luxation episode in patients evaluated by computed axial tomography (CAT), and in 86% of the recurrences - the latter being proportional to the magnitude of the lesion(10). Dickens et al. likewise observed this same association, though with a lesser frequency: a 6.8% incidence of glenoid bone lesions after a first luxation episode, and a total bone loss of 22.8% in the recurrences(11).
Lastly, it should be mentioned that the risk factors exert a synergic additive effect: the sum of two relevant risk factors such as glenoid bone lesion and young patient age results in an even higher relapse rate (72%)(12).
Case history in the event of suspected glenohumeral instability
When glenohumeral instability is suspected, the case history seeks to help confirm the diagnosis and establish the degree of severity of the condition.
The main symptom must be identified in order to establish a correct diagnosis: luxation, subluxation, pain, impaired sports performance, etc. We also need to know what position of the arm causes appearance of the symptoms, the location of the pain (if any), and whether the symptoms manifest at rest or during sleep. It is important to determine whether there is a lesional mechanism (traumatic or not), or a triggering action.
Among other aspects, we must investigate the circumstances of the first luxation episode, whether assisted reduction proved necessary, and whether there is imaging confirmation of the dislocated shoulder. We also must ask about the number of previous dislocations, the age at onset, whether the patient participates in contact sports, whether he or she has undergone previous surgeries of that shoulder, limb dominance, luxations of other joints, voluntariness in reproducing the symptoms, etc. - since these are all risk factors for relapse after treatment.
Physical examination
Exploration involves performing a series of manoeuvres that trigger the clinical manifestations described by the patient. We must identify the direction of instability (anterior, posterior, inferior), whether it is unidirectional, bidirectional or multidirectional, and the presence of hyperlaxity and/or dyskinesia of the scapula-thorax, and must also discard associated lesions.
The present article describes the main exploratory manoeuvres related to anterior instability and hyperlaxity.
Manoeuvres for exploring anterior instability
Apprehension test
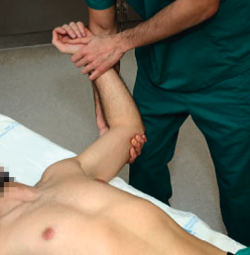
The patient is placed in supine decubitus on the table, with the explored arm free and outside the table. The explorer takes the arm of the patient to 90º of abduction, with the elbow flexed and, starting from a neutral rotation position, external rotation is gradually increased (Figure 1). If this produces patient apprehension and pain, anterior instability can be assumed with a sensitivity of 72% and a specificity of 96%(13). In some cases the manoeuvre only produces pain, with no real instability sensation. In such situations the pain can cause some confusion, and may suggest the presence of anterior shoulder instability - though we need more positive specific test results in order to confirm this, such as the relocation test described below.
Jobe relocation or re-centring test
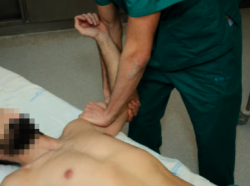
Starting from the position of the positive apprehension manoeuvre, the explorer places the hand on the anterior part of the proximal half of the arm, applying force in a posterior direction, in order to re-centre the humeral head in the glenoid cavity; this test proves positive if the pain and apprehension disappear and external rotation can be increased without symptoms (Figure 2). This test allows us to distinguish between purely painful shoulders without instability (such patients will have a previous positive apprehension test and a negative relocation test) and shoulders with pain due to instability (in which both the apprehension and the relocation test will be positive), and may indicate damage to the labrum(14,15). The sensitivity of this test is 30-81%, with a specificity of 90-92%(13).
Release/surprise test
Starting from a positive relocation test and from that same position, the examiner suddenly and without warning, stops pressing the extremity in a posterior direction, and the humeral head then rapidly and spontaneously returns to its forced anterior position. If the manoeuvre again triggers pain sensation and/or apprehension, it will be considered positive for anterior instability(15). The sensitivity of this test is 63.89%, with a specificity of 98.91%(15).
The combination of positivity with the three above described tests has shown a high positive predictive value (93.6%) and a high negative predictive value (71.9%)(15).
Manoeuvres for exploring shoulder laxity
The following tests allow us to identify excess translation or rotation due to laxity of the capsule-ligament structures, which is not necessarily indicative of disease. In fact, no abnormality is assumed if such translation or rotation is asymptomatic, isolated and bilateral.
Anterior drawer test
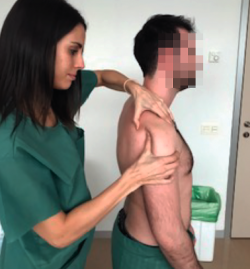
This test involves pushing the humeral head anteriorly with the hand while the other hand is used to grasp and block the scapula (Figure 3). It is considered to be positive if easy excessive anterior displacement of the humeral head is evidenced, compared with the contralateral shoulder. Three grades have been established according to the displacement of the humeral head with respect to the glenoid cavity: type 1 (translation reaches the anterior glenoid margin); type 2 (translation exceeds the glenoid margin); and type 3 (translation exceeds the glenoid margin and the humeral head is not spontaneously reduced). This test is indicative of excessive laxity of the anterior capsule-ligament structures and has a sensitivity of 53-58% and a sensitivity of 85-93%(16).
Load and shift test
With the patient lying down, a hand is placed on the distal portion of the arm, and the other hand on the proximal portion. Starting from a position of 20º of abduction and flexion to centre the humeral head in the glenoid cavity, force is applied in the axial direction towards the cavity and performing anterior translation of the humeral head. This test is positive if - as in the drawer test - we detect excessive humeral translation, and indicates excessive anterior laxity. The sensitivity of this test is 72%, with a specificity of 90%(16).
Sulcus test or sign
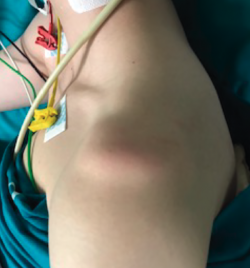
In this test, longitudinal arm traction is exerted in a downward direction. The test is positive if the humeral head displaces inferiorly and a sulcus or groove appears between the acromion and the humeral head(17), reflecting inferior laxity (redundant inferior capsule recess and both inferior glenohumeral ligaments) (Figure 4). A positive bilateral and asymptomatic test is not considered pathological.
Depending on the size of the sulcus, laxity can be classified as type I (< 1 cm), type II (1-2 cm) or type III (> 2 cm).
Gagey test
Hyperabduction test: the scapula is stabilized with one hand and the other is used to abduct the arm of the patient. Excessive abduction above 105º is suggestive of hyperlaxity(17). A difference of over 20º with respect to the contralateral shoulder is suggestive of insufficiency of the inferior glenohumeral ligament. The test in all cases should be performed bilaterally, comparing both shoulders.
It is important to clarify that the presence of shoulder laxity is not synonymous of hyperlaxity. The diagnosis of hyperlaxity also requires its presence in other joints, and criteria such as those of Beighton likewise must be met(18).
Apart from the mentioned tests, we must include all those manoeuvres designed to detect hyperlaxity, scapulothoracic dyskinesia and neurological or rotator cuff lesions that might be associated, and whose description falls beyond the scope of the present guide.
Imaging tests and diagnostic algorithm
Plain radiographs
Plain radiography is the first technique to be used for the diagnostic assessment of glenohumeral instability, and in some cases it is the only tool needed.
Anteroposterior (AP) projections in the scapular and axillary plane are basic for assessing adequate joint congruence, the joint space and the presence of possible bone lesions of the humeral head (Hill-Sachs or McLaughlin lesions) and glenoid cavity. It is advisable to add AP projections with rotations and scapular projection in Y to better visualize the tuberosities, coracoid process, acromion and bone defects(19).
Small Hill-Sachs lesions (HSLs) in AP projection are only visualized with the shoulder in internal rotation (Figure 5A and B), while larger lesions can be seen in both internal rotation and external rotation(20) - the axillary projection affording better visualization of HSLs, however. For assessing glenoid bone loss, it is advisable to use an axillary projection or modified Bernageau projection, with a sensitivity and specificity of over 90%(20,21).
reaca.28272.fs2009049en-figure5.png
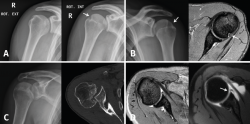
Figure 5. A and B: two cases of patients with Hill-Sachs lesions. A: comparison showing that it is easier to visualize the defect with the shoulder in internal rotation, especially in the case of a small defect; B: the same Hill Sachs lesion is observed on a comparative basis in the plain radiograph and MRI scan; C: computed axial tomography (CAT) view of a reverse Hill-Sachs lesion; D: ALPSA type lesion in conventional magnetic resonance imaging (MRI) and arthro-MRI. The latter technique is useful in the event of diagnostic doubt, since it offers more precise information about the type and characteristics of the capsulolabral lesion.
If these bone lesions are visible on plain radiographs, they can be assumed to be of considerable size; a CAT study therefore would be indicated to quantify the defect.
Computed axial tomography
This technique is considered to be the gold standard for the identification of bone defects. The use of CAT would be indicated in the presence of bone lesions identified or strongly suspected from the plane radiographs.
Three-dimensional reconstructions afford valuable information about the morphology of the glenoid cavity, as well as precise measurement of the size, location and depth of the defects of the humeral head (Figure 5C) and glenoid cavity(19,22) - these being decisive factors for the planning of surgery.
Magnetic resonance imaging (MRI)
This is the technique of choice for assessing soft tissue and cartilage lesions. It is indicated in cases of relapsing instability, in first luxation episodes with a high risk of relapse, and in cases with suspected associated lesions (rotator cuff, chondral lesions, etc.). Magnetic resonance imaging allows us to analyse the presence and extent of capsulolabral(23) and joint lesions.
It is known that the incidence of rotator cuff lesions in patients with shoulder instability increases with age, having been described in 40% of all cases between 40-55 years of age, in 71% between 56-70 years of age, and in up to 100% of the cases in individuals over age 70(24). This would justify the use of MRI in patients over 40 years of age, with a view to discarding rotator cuff lesions.
Ultrasound
Ultrasound is very useful, since it is rapid and easy to use, and allows us to rule out rotator cuff lesions particularly in patients at risk of having such lesions (individuals over 40 years of age), and in subjects in which rotator cuff damage is clinically suspected.
Arthro-magnetic resonance imaging (arthro-MRI)
Arthro-MRI with intraarticular gadolinium contrast offers greater sensitivity and specificity in the diagnosis of lesions of the labrum than conventional MRI without contrast injection(19), though it is not a necessary routine test provided the MRI images are analysed by an experienced professional. It is reserved for those cases in which conventional MRI poses doubts (Figure 5D), or in patients with postsurgical relapse.
Diagnostic tests protocol
The protocol for requesting the above described diagnostic tests varies depending on whether the patient presents a first dislocation episode or only suspected instability.
Following a first episode of glenohumeral dislocation, we must start the study with basic radiological projections - fundamentally an AP view and an axillary view. These tests are able to discard the presence of bone lesions of the humeral head and glenoid cavity. If lesions are identified, the next step would be a CAT scan to evaluate them. Ultrasound can be used as an initial screening option to rule out associated damage to the rotator cuff in patients at risk due to age. Magnetic resonance imaging is not necessary on a routine basis following a first episode, unless the patient is at high risk of relapse and we need to expand the study to define a surgical plan, or the patient has suspected or previously ultrasound-confirmed rotator cuff damage (Figure 6).
The study protocol will differ in the case of symptomatic recurrent instability, where the aim will be to identify the underlying cause, precisely establish the type of lesions to allow correct surgical planning where indicated, and rule out associated lesions. We must start the study with good radiological projections in the form of an AP view and an axillary view, and with an MRI scan. This will allow us to diagnose damage to the labrum, capsule-ligament structures and chondral, bone and tendon elements.
If bone lesions are evidenced from the plain radiographs and/or MRI scan, a CAT study is advisable in order to evaluate them (Figure 6).
In more complex scenarios such as relapse or cases where MRI generates doubts and the study needs to be expanded, we can resort to arthro-MRI.
Relevance of bone defects in anterior glenohumeral instability
Bone defects of the glenoid cavity
The relevance of the study of bone lesions of the glenoid cavity lies in the need to quantify the magnitude of the bone defect, since this is a decisive factor for indicating surgery, i.e., for deciding whether to use a soft tissues technique or a technique involving bone grafting.
A range of techniques have been proposed for measuring bone defects of the glenoid cavity: plain radiographs(25), CAT(26), MRI(27,28) or arthroscopy(29). To date, the most widely accepted quantitative method for assessing bone defects is CAT. There are two main techniques: the best-fit circle method or the method of comparison with the contralateral shoulder; according to Jeske et al.(30), the difference in glenoid surface between the two anatomically normal shoulders in the same individual is barely 1.8%. Consequently, the contralateral shoulder can be used as reference in the absence of bone defects. If the contralateral shoulder also suffers glenohumeral instability, the best-fit circle method would be indicated(20).
Likewise, there are two ways to express the bone defect: by measuring the area or through lineal measurement of the length of the defect. In general, lineal measurement of the length of the defect is preferred, since it is simpler and requires no special software(31). With the three-dimensional (3D) reconstructions of both shoulders, we obtain a frontal view of the glenoid surface on which we must calculate the maximum horizontal distance (anteroposterior) of the glenoid cavity in both shoulders. The glenoid defect (d) would be defined as the difference: maximum horizontal distance of the healthy contralateral shoulder (D) minus the maximum horizontal distance of the affected shoulder. The measure of this bone defect (d) is expressed as a percentage: d / D × 100 (%).
Defining the maximum bone defect amenable to Bankart repair with a low risk of relapse is not easy. Different limits have been established over time by different authors. Classically, a glenoid bone surface defect of 25% has been regarded as the limit for the use of soft tissue techniques for the management of instability. Above this limit we would need bone grafting techniques such as the Latarjet procedure or bone stop with iliac crest graft or allograft(32,33). According to Itoi, a defect of 21% in superoinferior length or 28% of the width of the glenoid cavity is the limit beyond which the failure rate of soft tissue repair increases(34,35).
The above is valid for unipolar defects of the glenoid surface (on an isolated basis). However, most patients present combined bone defects affecting both the glenoid cavity and the humeral head(33). In these cases, smaller defects of the glenoid cavity combined with humeral head defects can cause failure in soft tissue repair procedures and instability relapse.
In general, many authors(31,36,37) agree that a glenoid defect of 25% together with the on-track and off-track concept of HSLs define the limits for using a soft tissue technique or bone stop procedure. Other authors have reconsidered lowering the limit of the glenoid defect to 20%. In general, increasingly lower glenoid defects are being accepted or considered as the limit. Some authors, such as Jeon et al.(38), define the limit as an anterior glenoid defect of 15-20%, and in these cases they obtain better results with the Latarjet technique than with Bankart soft tissue procedures (better outcomes in terms of fewer relapses with Latarjet: 6.5% versus 22.5% with Bankart repair; p = 0.040)(38). In another study, Saha et al.(39), reported that "critical" bone loss should be below the 20-25% threshold often cited. In their study, involving a military population with demanding activity, a bone loss of over 13.5% resulted in poorer outcomes(39). Likewise, S. J. Shin et al.(40) concluded that the critical level of anterior glenoid bone loss at which bone restorations should be considered is closer to 15%(40).
Thus, no concrete standardized and universal limit has been established. Nevertheless, the most current literature does seem to coincide that glenoid cavity bone defects of between 15-20% must be taken into account - not only defects beyond 25% as affirmed in older publications. No concrete protocol can be found in the literature; rather, the recommendation is to individualize each case with bone defects in that range, studying it globally together with the rest of the lesions and risk factors, without underestimating bone lesions exceeding 15% of the glenoid cavity.
In conclusion, according to most of the consulted current literature(38,39,40), and as general recommendations for our work, in patients presenting bone defects that exceed 20% of the glenoid cavity, we should consider procedures combining anterior bone grafting - since doing so considerably reduces the risk of relapse. In the case of bone defects between 15-20%, it is preferable to individualize each case, globally taking into account all the risk factors for relapse in the patient, since these may condition a more aggressive approach with smaller defects.
Bone defects of the humeral head
The prevalence of HSLs is about 65% following first dislocation, and between 84-93% after recurrent dislocations(33,41).
These lesions produce instability in abduction movements combined with external rotation, since it is in this moment when the posterolateral zone of the humeral head comes into contact with the glenoid cavity. If the lesion is completely contained within the cavity, instability will not result; however, if part of the lesion is not covered, it can engage with the anterior margin of the glenoid cavity and cause luxation. This is known as an engaging Hill-Sachs lesion(42).
The concepts of "engaging lesion" and "glenoid track" (GT) are complementary and consistent with each other, since both evaluate the presence of a humeral bone defect and its interaction with the glenoid cavity over the range of motion. The GT is the area of the posterior joint surface of the humerus in contact with the glenoid cavity with the arm in maximum abduction and external rotation(31). The GT at 90° of abduction has been established as 83% of the width of the glenoid surface(42). In order to determine whether an HSL is within the GT and is on-track or off-track, it is important to know its spatial positioning with the shoulder in abduction and the distance between the margin of the joint surface (medial margin of the GT) and the medial margin of the insertion of the rotator cuff in the humeral head.
The importance of this concept lies in its clinical implications. The presence of off-track HSL is associated to increased recurrence after surgery (33-75%) compared with on-track lesions (6-8%) when treatment is limited to Bankart repair(43), and has been identified as an independent relapse risk factor(44). In bipolar lesions, since there is damage to the glenoid surface, a decrease in GT is observed; consequently, HSLs that isolatedly would be on-track lesions, can become off-track lesions on combining with glenoid cavity damage(33). The formula used to determine the GT of the affected shoulder is to calculate 83% of the maximum horizontal distance of the contralateral glenoid cavity (normal GT) minus the glenoid defect (pathological GT = normal GT − d)(36). Then, over the posterior view of the humeral head, we calculate the humeral defect (HD): diameter of the HSL + bone bridge between the lateral margin of the HSL and the medial margin of the rotator cuff (HD = Hill-Sachs + bone bridge)(36).
In order to determine whether the lesion is on-track or off-track, we subtract HD from GT (GT − HD). In this way, if HD is greater than GT, the lesion will be off-track, and stabilization based on isolated Bankart repair will be at risk of failure (HD > GT → off-track). In contrast, if HD is smaller than GT, the lesion will be on-track and stabilization based on isolated Bankart repair would suffice (HD < GT → on-track)(36).
The manoeuvre associated to Bankart repair in cases of off-track HSL is remplissage - a technique that will be described further below.
Conservative management
Conservative management following a first episode of glenohumeral dislocation seeks to achieve healing of the damaged structures with a view to restoring stability and securing full and pain-free joint mobility(45).
Conservative management is indicated following a first glenohumeral dislocation episode in the absence of risk factors for relapse. In the presence of such factors, treatment should be individualized and decided on a consensus basis according to the particularities of each case(46). Conservative management a priori is not indicated in patients with multiple luxations (two or more), or in cases with established and symptomatic instability(47).
Treatment consists of immobilization for 1-3 weeks with a sling and adding analgesic or anti-inflammatory medication(48) - though there is no evidence that immobilization for more than 7-10 days significantly reduces the recurrence rate(49) - together with a phase-structured physiotherapeutic program(50).
The approach to patient rehabilitation after a first glenohumeral luxation episode should be based on the following phases(51,52):
- Protection phase. The objective here is to secure capsulolabral healing. The patient is to remain immobilized with the sling for the first 1-3 weeks, removing it only for passive shoulder mobilization, pendulum exercises, and active mobilization of the elbow, wrist and hand - in addition to isometric exercises of the deltoid muscle and scapular mobilization.
- Active recovery phase. The objective here is to secure recovery of shoulder function. In this phase we introduce active and passive shoulder mobility exercises, scapular neuromuscular control and, posteriorly, strength-resistance exercises according to patient tolerance. It is advisable to strengthen the rotator cuff, which will help in affording stability, and restore infraspinatus - subscapularis muscle balance, together with periscapular muscle exercises.
- Functional recovery phase. Sports - occupational re-adaptation is the objective in this phase, which is characterized by the application of individualized recovery programs. Plyometric training is introduced in this period.
Surgical treatment of glenohumeral instability without bone defects
In the treatment of shoulder instability, the great glenohumeral joint mobility and its stability must be taken into account. When starting glenohumeral treatment, we must achieve a balance between joint stabilization and the stiffness derived from reduction of the motion range.
In the case of glenohumeral instability with no significant bone defect, capsulolabral repair (open or arthroscopic) has shown good functional outcomes with a low relapse rate(53,54,55). Progressive surgeon training in arthroscopic techniques has allowed this procedure to equal the performance of the open technique in terms of postoperative functional outcomes and relapse rate(56,57), minimizing the complications associated with greater aggression caused by the surgical approach(58).
Indications(46)
The main indications of surgical treatment are:
- Established and symptomatic glenohumeral instability (two or more luxation episodes).
- Symptoms of glenohumeral instability without manifest luxation (episodes of subluxation ± apprehension ± pain ± discomfort) with the presence of a labral lesion.
- First glenohumeral luxation episode with high risk of recurrence (age, competition contact sports, significant bone defects)(46,59).
Here again, these indications always must be conditioned to the requirements and characteristics of each patient, with individualization of each therapeutic decision.
Surgical technique
Arthroscopic capsulolabral repair
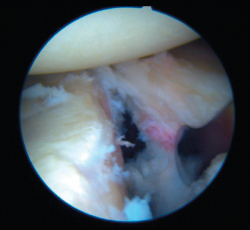
Bankart lesion consists of deinsertion of the glenohumeral ligaments and labrum in its anteroinferior portion. It is the most common type of lesion in cases of anterior instability and is moreover present after a first luxation episode in 90% of all traumatic dislocations. Instability is usually a consequence of failure of these lesions to heal, or of healing in an anomalous position, usually in the form of medialization over the neck of the glenoid cavity.
Arthroscopic Bankart repair is regarded as the first option for the treatment of traumatic, unidirectional, anteroinferior and inferior instability of the shoulder. Surgical treatment involves labrum release and mobilization, allowing it to be reinserted in its correct position. Such release and correction of the position of the labrum proves technically more complex in ALPSA (anterior labroligamentous periosteal sleeve avulsion) and Perthes type lesions, but is essential in order to ensure effective repair of the labrum. In this way, Bankart repair affixes the labrum to the margin of the glenoid cavity and ascends the capsule and ligaments proximally, providing them with tension.
Description of the technique
The procedure is generally performed under general anesthesia and brachial plexus block. It is advisable to carry out an exploration of the shoulder under anesthesia, since this allows us to evaluate the glenohumeral joint with muscle relaxation and clinically confirm the instability, as well as its type and grade.
The patient may be placed in a deck chair or in lateral decubitus, depending on the preference of the surgeon. We start by inserting a 30º arthroscope through a standard vision posterior port. Systematic exploration of the glenohumeral joint is very important for establishing a firm diagnosis of all the lesions, evaluating possible detachments of the anterior labrum, capsular tears, upper labral lesions and biceps lesions, bone damage including Bankart bone lesions and HSLs, rotator cuff disease and alterations of the inferior part of the capsular recess in order to discard humeral avulsion of the inferior glenohumeral ligament.
Then, an anteroinferior port is established using an outside-inside technique with a spinal needle. The exact point of the port will be that allowing us to reach the anteroinferior labrum (6 o'clock position), normally close to the upper subscapular margin and with a direction from lateral to medial. It is advisable to use cannulas in all ports. In the mentioned port, we commonly use a cannula 8 mm in diameter, since it will constitute the working port.
Lastly, the accessory anterosuperior port is placed at the most lateral and upper point of the rotator interval and close to the bicipital groove, allowing us to work on either side of the biceps. This port improves anterior visualization of the labrum and facilitates suture recovery.
Once the Bankart lesion has been confirmed, we detach and mobilize the capsulolabral complex from the glenoid neck with arthroscopic rasps and/or dissectors until satisfactory and subscapular fibers are visualized (Figure 7). Before implant placement, it is necessary to perform decortication of the anterior glenoid neck using a 4-mm synoviotome. Repair is started after this step, always working from lower to upper.
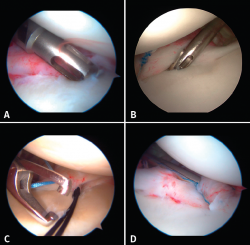
The implants are to be placed at 45º from the joint surface adjacent to the margin of the anterior cartilage, taking care not to medialize them over the neck of the glenoid cavity (Figures 8A and B). We start with the lowest implant; depending on the extent of the lesion, we will need 3, 4 or 5 implants spaced 3-5 mm apart.
Capsulolabral repair can be carried out in different ways, since a number of suture passer systems are available: indirect nitinol devices (these require two steps, but are less damaging to the tissues) or direct passer forceps (involving a single step, but causing more damage).
If a direct system is used, we pass direct penetrating-suture retrieval forceps from anterior to posterior, tunnelling the tissue to be sutured and taking one of the implant threads for knotting to the post. This in all cases will be the thread passing through the tissue, and we try to keep the knot of the thread anterior to the reconstructed tissue without friction against the humeral joint surface. This same step can be made with indirect passer forceps, tunnelling a nitinol wire through the tissue (Figure 8C), allowing us to grasp and then knot one of the implant threads (Figure 8D).
The use of knotless implants spares us this knotting step, since these are systems in which the thread is threaded and blocked within the implant. These implants avoid the risk of loosening of the knot and loss of tension, as well as the risk of a prominent knot that may be mobilized and produce friction against the joint surface.
Presence of associated lesions
The presence of residual plastic deformity of the anteroinferior capsule or hyperlaxity of the underlying capsule has been related to an increased relapse rate due to insufficiency of the capsule-ligament complex (anterior bundle of the inferior glenohumeral ligament). Capsule plicature would be indicated in these cases, involving inclusion of the inferior capsule in passing the suture, in an attempt to encompass it within the repair. In this way we are able to ascend the capsule and inferior ligaments proximally, securing greater tension of these elements and obtaining greater repair stability.
The HAGL (humeral avulsion of the glenohumeral ligaments) lesion is characterized by avulsion of the inferior glenohumeral ligament on the humeral side. Anterior HAGL (deinsertion of the anterior inferior glenohumeral ligament) is more common than posterior HAGL. In some cases there may be a bone fragment from the medial portion of the humeral neck (bony HAGL). These lesions in themselves may generate symptomatic glenohumeral instability without the presence of a capsulolabral lesion(60).
Such lesions are uncommon (1-10%) and are greatly underdiagnosed. They are usually diagnosed by arthroscopy, and go undetected in the MRI study. If these lesions go undetected and are not adequately treated, they may cause luxation relapse(61). The treatment of these lesions consists of reaffixing the capsule and glenohumeral ligaments to their anatomic humeral insertion by means of implants. Many techniques for the repair of such lesions have been described, according to the number and type of anchorings used, the order to be followed in repair, and the type of knot used(62). Here again, the type of lesion and the patient characteristics require an individualized decision regarding the most convenient repair technique in each case.
In the case of complete rupture of the inferior glenohumeral ligament or insufficient joint capsule, treatments involving open ligamentoplasties or even bone stop techniques are preferred. Mikel Sánchez et al.(63) described an arthroscopy assisted technique for reconstruction of the inferior glenohumeral ligament involving artificial capsular reinforcement in 167 patients, with satisfactory results.
Bankart associated HSLs are found in a large percentage of cases (71-100%); we must distinguish whether the lesion is an impingement lesion or not(33) or - if GT measurement has been used to globally assess the humeral and glenoid bone deficit - we must determine whether the lesion is on-track or off-track.
In the case of an impingement or off-track lesion, the risk of relapse increases considerably(33); in these cases the required surgical manoeuvre should convert the HSL into an on-track lesion again or into a non-impingement lesion. The remplissage technique has been successfully used for this purpose(64) and involves fixation of the posterior capsule and part of the infraspinatus tendon (capsulotenodesis) to the bone defect of the humeral head. Connolly et al.(65) proposed this technique as an open procedure four decades ago, though it was Wolf in 2004 who first described the arthroscopic technique as a modification of the open procedure. Many authors have reported good outcomes combining arthroscopic Bankart repair with remplissage. Wolf et al.(66) reported their findings after up to 10 years of follow-up, with the recording of recurrence in only two out of 45 patients (4.4%). Boileau et al.(61) used this procedure in 47 of 459 shoulders, and only one shoulder (2.1%) suffered recurrent instability. Recent systematic reviews have found the overall recurrence rate after combining both techniques to range from 3.4% to 5.4%, without important joint balance restrictions(67,68). As an undesired effect, the technique may produce a certain decrease in mobility, especially referred to external rotation, though it considerably lessens the instability recurrence rate(61).
Rotator cuff lesions increase in frequency with age (80% in patients over 60 years of age). Therefore, we must always suspect such injuries in individuals over age 40 or with rotator cuff clinical manifestations; in these cases MRI or ultrasound would be indicated for diagnosis and treatment.
Complications
The most frequent complication is recurrence. The relapse rate reported by most studies following Bankart repair is between 6-12.5%(57,59), and this rate is inversely proportional to patient age (both age at the time of surgery and age at which instability first appears) and directly proportional to the number of previous luxations and the degree of involvement of the shoulder anatomy after successive luxations (plastic deformity of the capsule, involvement of the muscle surrounding the shoulder, etc.)(70). Both younger age and the number of luxations prior to surgery are considered to be the most relevant risk factors for recurrence following surgery(71).
Stiffness is a frequent complication more closely related to open approaches and the first arthroscopic treatments with non-reabsorbable implants; the most commonly reported loss of motion range corresponds to external rotation. The overall incidence of stiffness following arthroscopic Bankart repair ranges from 1.6%(72) to 5%(73). Most patients with stiffness benefit from conservative management, with the possibility of mobilization under anesthesia and/or arthroscopic capsulotomy in the event of no improvement after 6 months(74). In cases with isolated severe loss of external rotation, arthroscopic treatment has been proposed with release of the rotator interval and of the adherences between the subscapularis tendon and neck of the glenoid cavity(75).
The main infectious complications have been superficial infections of the arthroscopy ports. The reported deep infections rate is 0.22%, and the most common pathogens are staphylococci (S. aureus and S. epidermidis). When infection is confirmed, it is advisable to perform early joint cleansing with the collection of culture samples and the start of antibiotic treatment as soon as possible(63).
Another complication is chondrolysis, which is characterized by rapid destruction of joint cartilage secondary to destruction of the chondrocytes and dissolution of the cartilage matrix. This complication has been described in thermal capsulorrhaphy. It has also been reported in 50% of the cases in which infiltration was made with bupivacaine (0.5%) using a high-flow intraarticular pain pump catheter in the postoperative period(76,77).
Although very uncommon, there have been reports of nerve damage (0.3%)(78). The axillary nerve is the most frequently affected nerve. It runs anterior to the subscapular muscle and rests on the inferolateral margin of the subscapular tendon; damage to this nerve is caused by capsulolabral reconstruction in the anteroinferior zone (between the 5 and 6 o'clock positions). Once nerve damage has been identified, we must wait 3-6 months, with the possibility of surgical exploration if no improvement is observed after this period. There have also been reports of transient nerve damage to the brachial plexus and ulnar nerve, of a self-limiting nature(74).
Conclusions
Traumatic glenohumeral instability is particularly common in the young and physically active population. The case history should seek to correctly identify the problem and the risk factors for relapse after treatment. Complementary tests should indicate the origin of the lesion and quantify it. In the absence of significant bone defects, i.e., a glenoid bone defect of under 20% or under 15% if there are other risk factors, arthroscopic capsulolabral repair is a safe, reproducible and effective treatment option.
Figuras
Figure 5. A and B: two cases of patients with Hill-Sachs lesions. A: comparison showing that it is easier to visualize the defect with the shoulder in internal rotation, especially in the case of a small defect; B: the same Hill Sachs lesion is observed on a comparative basis in the plain radiograph and MRI scan; C: computed axial tomography (CAT) view of a reverse Hill-Sachs lesion; D: ALPSA type lesion in conventional magnetic resonance imaging (MRI) and arthro-MRI. The latter technique is useful in the event of diagnostic doubt, since it offers more precise information about the type and characteristics of the capsulolabral lesion.
Información del artículo
Cita bibliográfica
Autores
Andrea Paniagua González
Unidad de Hombro y Codo. Hospital Fraternidad-Muprespa Habana. Madrid
Unidad de Hombro y Codo. Hospital Universitario Ramón y Cajal. Madrid
Lorena Trueba Sánchez
Hospital Mompía. Santander, Cantabria
Cirugía Ortopédica y Traumatología. Hospital Universitario de Burgos
Noelia Alonso García
Complejo Asistencial de Segovia
Alfonso Vaquero Picado
Servicio de Cirugía Ortopédica y Traumatología A, Hospital Universitario La Paz, Madrid, España
Jorge García Donaire
Hospital Cenyt. Estepona, Málaga
Maximiliano Sánchez Martos
Hospital Universitario Virgen de Valme. Sevilla
Javier Ruiz Diaz
Ibermutua. Oviedo
Hospital Ibermutua Asturias
Gonzalo Hernández Fernandez
Hospital Central de la Defensa Gómez Ulla. Madrid
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved studies in humans or in animals.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Owens BD, Nelson BJ, Duffey ML, Mountcastle SB, Taylor DC, Cameron KL, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-11.
-
2Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am. 2010;92(3):542-9.
-
3Rockwood CA. The shoulder. Philadelphia: Saunders/Elsevier; 2009.
-
4Olds M, Ellis R, Donaldson K, Parmar P, Kersten P. Risk factors which predispose first-time traumatic anterior shoulder dislocations to recurrent instability in adults: a systematic review and meta-analysis. Br J Sports Med. 2015;49(14):913-22.
-
5Hayes K, Callanan M, Walton J, Paxinos A, Murrell GA. Shoulder instability: management and rehabilitation. J Orthop Sports Phys Ther. 2002;32(10):497-509.
-
6Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38-A(5):957-77.
-
7Robinson CM, Howes J, Murdoch H, Will E, Graham C. Functional outcome and risk of recurrent instability after primary traumatic anterior shoulder dislocation in young patients. J Bone Joint Surg Am. 2006;88(11):2326-36.
-
8Hovelius L, Olofsson A, Sandstrom B, Augustini BG, Krantz L, Fredin H, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-52.
-
9Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-7.
-
10Griffith JF, Antonio GE, Yung PS, Wong EM, Yu AB, Ahuja AT, et al. Prevalence, pattern, and spectrum of glenoid bone loss in anterior shoulder dislocation: CT analysis of 218 patients. AJR Am J Roentgenol. 2008;190(5):1247-54.
-
11Dickens JF, Slaven SE, Cameron KL, Pickett AM, Posner M, Campbell SE, et al. Prospective Evaluation of Glenoid Bone Loss After First-time and Recurrent Anterior Glenohumeral Instability Events. Am J Sports Med. 2019;47(5):1082-9.
-
12Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-84.
-
13Farber AJ, Castillo R, Clough M, Bahk M, McFarland EG. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88(7):1467-74.
-
14Bushnell BD, Creighton RA, Herring MM. The bony apprehension test for instability of the shoulder: a prospective pilot analysis. Arthroscopy. 2008;24(9):974-82.
-
15Lo IK, Nonweiler B, Woolfrey M, Litchfield R, Kirkley A. An evaluation of the apprehension, relocation, and surprise tests for anterior shoulder instability. Am J Sports Med. 2004;32(2):301-7.
-
16Hippensteel KJ, Brophy R, Smith MV, Wright RW. Comprehensive Review of Provocative and Instability Physical Examination Tests of the Shoulder. J Am Acad Orthop Surg. 2019;27(11):395-404.
-
17Eshoj H, Ingwersen KG, Larsen CM, Kjaer BH, Juul-Kristensen B. Intertester reliability of clinical shoulder instability and laxity tests in subjects with and without self-reported shoulder problems. BMJ Open. 2018;8(3):e018472.
-
18Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-53.
-
19Cain EL Jr, Ryan MK. Traumatic Instability: Treatment Options and Considerations for Recurrent Posttraumatic Instability. Sports Med Arthrosc Rev. 2018;26(3):102-12.
-
20Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord. 2015;16:164.
-
21Pansard E, Klouche S, Billot N, Rousselin B, Kraus TM, Bauer T, et al. Reliability and validity assessment of a glenoid bone loss measurement using the Bernageau profile view in chronic anterior shoulder instability. J Shoulder Elbow Surg. 2013;22(9):1193-8.
-
22Sugaya H. Techniques to evaluate glenoid bone loss. Curr Rev Musculoskelet Med. 2014;7(1):1-5.
-
23Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-83.
-
24Simank HG, Dauer G, Schneider S, Loew M. Incidence of rotator cuff tears in shoulder dislocations and results of therapy in older patients. Arch Orthop Trauma Surg. 2006;126(4):235-40.
-
25Garth WP Jr, Slappey CE, Ochs CW. Roentgenographic demonstration of instability of the shoulder: the apical oblique projection. A technical note. J Bone Joint Surg Am. 1984;66(9):1450-3.
-
26Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-30.
-
27Huijsmans PE, Haen PS, Kidd M, Dhert WJ, van der Hulst VP, Willems WJ. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: a cadaveric study. J Shoulder Elbow Surg. 2007;16(6):803-9.
-
28Omori Y, Yamamoto N, Koishi H, Futai K, Goto A, Sugamoto K, et al. Measurement of the Glenoid Track In Vivo as Investigated by 3-Dimensional Motion Analysis Using Open MRI. Am J Sports Med. 2014;42(6):1290-5.
-
29Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18(5):488-91.
-
30Jeske HC, Oberthaler M, Klingensmith M, Dallapozza C, Smekal V, Wambacher M, et al. Normal glenoid rim anatomy and the reliability of shoulder instability measurements based on intrasite correlation. Surg Radiol Anat. 2009;31(8):623-5.
-
31Itoi E. 'On-track' and 'off-track' shoulder lesions. EFORT Open Rev. 2017;2(8):343-51.
-
32Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res. 2002(400):65-76.
-
33Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-94.
-
34Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
-
35Yamamoto N, Muraki T, Sperling JW, Steinmann SP, Cofield RH, Itoi E, et al. Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Joint Surg Am. 2010;92(11):2059-66.
-
36Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy. 2014;30(1):90-8.
-
37Di Giacomo G, Piscitelli L, Pugliese M. The role of bone in glenohumeral stability. EFORT Open Rev. 2018;3(12):632-40.
-
38Jeon YS, Jeong HY, Lee DK, Rhee YG. Borderline Glenoid Bone Defect in Anterior Shoulder Instability: Latarjet Procedure Versus Bankart Repair. Am J Sports Med. 2018;46(9):2170-6.
-
39Shaha JS, Cook JB, Song DJ, Rowles DJ, Bottoni CR, Shaha SH, et al. Redefining "Critical" Bone Loss in Shoulder Instability: Functional Outcomes Worsen With "Subcritical" Bone Loss. Am J Sports Med. 2015;43(7):1719-25.
-
40Shin SJ, Koh YW, Bui C, Jeong WK, Akeda M, Cho NS, et al. What Is the Critical Value of Glenoid Bone Loss at Which Soft Tissue Bankart Repair Does Not Restore Glenohumeral Translation, Restricts Range of Motion, and Leads to Abnormal Humeral Head Position? Am J Sports Med. 2016;44(11):2784-91.
-
41Spatschil A, Landsiedl F, Anderl W, Imhoff A, Seiler H, Vassilev I, et al. Posttraumatic anterior-inferior instability of the shoulder: arthroscopic findings and clinical correlations. Arch Orthop Trauma Surg. 2006;126(4):217-22.
-
42Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-56.
-
43Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical Validation of the Glenoid Track Concept in Anterior Glenohumeral Instability. J Bone Joint Surg Am. 2016;98(22):1918-23.
-
44Lee SH, Lim KH, Kim JW. Risk Factors for Recurrence of Anterior-Inferior Instability of the Shoulder After Arthroscopic Bankart Repair in Patients Younger Than 30 Years. Arthroscopy. 2018;34(9):2530-6.
-
45Boffano M, Mortera S, Piana R. Management of the first episode of traumatic shoulder dislocation. EFORT Open Rev. 2017;2(2):35-40.
-
46Ávila Lafuente JL, Moros Marco S, García Pequerul JM. Controversies in the Management of the First Time Shoulder Dislocation. Open Orthop J. 2017;11:1001-10.
-
47Handoll HH, Hanchard NC, Goodchild L, Feary J. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2006(1):CD004962.
-
48De Carli A, Vadala AP, Lanzetti R, Lupariello D, Gaj E, Ottaviani G, et al. Early surgical treatment of first-time anterior glenohumeral dislocation in a young, active population is superior to conservative management at long-term follow-up. Int Orthop. 2019;43(12):2799-805.
-
49Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-33.
-
50Ruiz Ibán MA, Díaz Heredia J, García Navlet M, Serrano F, Santos Oliete M. Multidirectional Shoulder Instability: Treatment. Open Orthop J. 2017;11:812-25.
-
51Bateman M, Smith BE, Osborne SE, Wilkes SR. Physiotherapy treatment for atraumatic recurrent shoulder instability: early results of a specific exercise protocol using pathology-specific outcome measures. Shoulder Elbow. 2015;7(4):282-8.
-
52Wilk KE, Macrina LC. Nonoperative and postoperative rehabilitation for glenohumeral instability. Clin Sports Med. 2013;32(4):865-914.
-
53Adam M, Attia AK, Alhammoud A, Aldahamsheh O, Al Ateeq Al Dosari M, Ahmed G. Arthroscopic Bankart repair for the acute anterior shoulder dislocation: systematic review and meta-analysis. Int Orthop. 2018;42(10):2413-22.
-
54Mahure SA, Mollon B, Capogna BM, Zuckerman JD, Kwon YW, Rokito AS. Risk factors for recurrent instability or revision surgery following arthroscopic Bankart repair. Bone Joint J. 2018;100-B(3):324-30.
-
55Kennedy MI, Murphy C, Dornan GJ, Moatshe G, Chahla J, LaPrade RF, et al. Variability of Reporting Recurrence After Arthroscopic Bankart Repair: A Call for a Standardized Study Design. Orthop J Sports Med. 2019;7(5):2325967119846915.
-
56Hobby J, Griffin D, Dunbar M, Boileau P. Is arthroscopic surgery for stabilisation of chronic shoulder instability as effective as open surgery? A systematic review and meta-analysis of 62 studies including 3044 arthroscopic operations. J Bone Joint Surg Br. 2007;89(9):1188-96.
-
57Petrera M, Patella V, Patella S, Theodoropoulos J. A meta-analysis of open versus arthroscopic Bankart repair using suture anchors. Knee Surg Sports Traumatol Arthrosc. 2010 Dec;18(12):1742-7.
-
58Williams HLM, Evans JP, Furness ND, Smith CD. It's Not All About Redislocation: A Systematic Review of Complications After Anterior Shoulder Stabilization Surgery. Am J Sports Med. 2019;47(13):3277-83.
-
59Boone JL, Arciero RA. First-time anterior shoulder dislocations: has the standard changed? Br J Sports Med. 2010;44(5):355-60.
-
60Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-6.
-
61Boileau P, O'Shea K, Vargas P, Pinedo M, Old J, Zumstein M. Anatomical and functional results after arthroscopic Hill-Sachs remplissage. J Bone Joint Surg Am. 2012;94(7):618-26.
-
62Provencher MT, McCormick F, LeClere L, Sánchez G, Golijanin P, Anthony S, et al. Prospective Evaluation of Surgical Treatment of Humeral Avulsions of the Glenohumeral Ligament. Am J Sports Med. 2017;45(5):1134-40.
-
63Moen TC, Rudolph GH, Caswell K. Complications of shoulder arthroscopy. J Am Acad Orthop Surg. 2014, 22(7):410-9.
-
64Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs "remplissage": an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-6.
-
65Connolly J, Regen E, Evans OB. The management of the painful, stiff shoulder. Clin Orthop Relat Res. 1972;84:97-103.
-
66Wolf EM, Arianjam A. Hill-Sachs remplissage, an arthroscopic solution for the engaging Hill-Sachs lesion: 2- to 10-year follow-up and incidence of recurrence. J Shoulder Elbow Surg. 2014;23(6):814-20.
-
67Alkaduhimi H, Verweij LPE, Willigenburg NW, van Deurzen DFP, van den Bekerom MPJ. Remplissage With Bankart Repair in Anterior Shoulder Instability: A Systematic Review of the Clinical and Cadaveric Literature. Arthroscopy. 2019;35(4):1257-66.
-
68Camus D, Domos P, Berard E, Toulemonde J, Mansat P, Bonnevialle N. Isolated arthroscopic Bankart repair vs. Bankart repair with "remplissage" for anterior shoulder instability with engaging Hill-Sachs lesion: a meta-analysis. Orthop Traumatol Surg Res. 2018;104(6):803-9.
-
69Harris JD, Gupta AK, Mall NA, Abrams GD, McCormick FM, Cole BJ, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-33.
-
70Olds MK, Ellis R, Parmar P, Kersten P. Who will redislocate his/her shoulder? Predicting recurrent instability following a first traumatic anterior shoulder dislocation. BMJ Open Sport Exerc Med. 2019;5(1):e000447.
-
71Wasserstein D, Dwyer T, Veillette C, Gandhi R, Chahal J, Mahomed N, et al. Predictors of dislocation and revision after shoulder stabilization in Ontario, Canada, from 2003 to 2008. Am J Sports Med. 2013;41(9):2034-40.
-
72Ahmed I, Ashton F, Robinson CM. Arthroscopic Bankart repair and capsular shift for recurrent anterior shoulder instability: functional outcomes and identification of risk factors for recurrence. J Bone Joint Surg Am. 2012;94(14):1308-15.
-
73Elmlund AO, Kartus J, Rostgard-Christensen L, Sernert N, Magnusson L, Ejerhed L. A 7-year prospective, randomized, clinical, and radiographic study after arthroscopic Bankart reconstruction using 2 different types of absorbable tack. Am J Sports Med. 2009;37(10):1930-7.
-
74Matsuki K, Sugaya H. Complications after arthroscopic labral repair for shoulder instability. Curr Rev Musculoskelet Med. 2015;8(1):53-8.
-
75Ando A, Sugaya H, Takahashi N, Kawai N, Hagiwara Y, Itoi E. Arthroscopic management of selective loss of external rotation after surgical stabilization of traumatic anterior glenohumeral instability: arthroscopic restoration of anterior transverse sliding procedure. Arthroscopy. 2012;28(6):749-53.
-
76Matsen FA 3rd, Papadonikolakis A. Published evidence demonstrating the causation of glenohumeral chondrolysis by postoperative infusion of local anesthetic via a pain pump. J Bone Joint Surg Am. 2013;95(12):1126-34.
-
77Scheffel PT, Clinton J, Lynch JR, Warme WJ, Bertelsen AL, Matsen FA 3rd. Glenohumeral chondrolysis: a systematic review of 100 cases from the English language literature. J Shoulder Elbow Surg. 2010;19(6):944-9.
-
78Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in Bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-9.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- <em>REACA</em>: school for authors
- Reconstruction of the medial patellofemoral ligament is effective in treating lateral patellofemoral instability, even in the presence of trochlear dysplasia. A review of 18 cases
- Results of the survey on the use of ultrasound among the members of the Spanish Association of Arthroscopy (Asociación Española de Artroscopia [AEA])
- Management of anterior glenohumeral instability without significant glenoid bone damage
- Rehabilitation following anterior capsulolabral arthroscopic Bankart repair: a guide for the young arthroscopist
- Diagnosis and management of injuries of the anterior cruciate ligament in skeletally immature patients. A narrative review
- Residual instability after reconstruction surgery of the anterior cruciate ligament. What are we missing?
- Posterolateral instability of the knee following osteochondral fracture including the femoral insertion of the popliteus tendon. A case report
- Meso-type long portion of the biceps: normal anatomical variant
- Instructions for authors (Apr. 2021)
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.