Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
Tendinopatía del supraespinoso: diagnóstico ecográfico y por resonancia magnética. Alternativas de tratamiento conservador y quirúrgico
Resumen:
La tendinopatía del supraespinoso es una causa frecuente de dolor y disfunción de la articulación del hombro. En el término tendinopatía se engloban las entidades patológicas tendinitis, tendinosis y lesiones de espesor parcial. La presencia de un mecanismo lesional compatible junto a una meticulosa exploración y pruebas de imagen complementarias son indispensables para llegar a un diagnóstico correcto. Existe una amplia variedad de modalidades de tratamiento para la tendinopatía del supraespinoso. Las alternativas no quirúrgicas incluyen la modificación de la actividad, los antiinflamatorios no esteroideos, la fisioterapia y diversas terapias físicas, así como variedad de infiltraciones. La cirugía está reservada para pacientes concretos cuando las opciones más conservadoras se han agotado y/o fracasado. La acromioplastia no es una alternativa adecuada para el manejo de estos pacientes. El objetivo de esta revisión es presentar las diferentes alternativas diagnósticas y terapéuticas para la tendinopatía del supraespinoso, dada la elevada prevalencia de dicha entidad patológica.
Abstract:
Supraspinatus tendinopathy is a common cause of shoulder joint dysfunction and pain. The term tendinopathy encompasses tendinitis, tendinosis and partial thickness injuries. The existence of a compatible mechanism of injury, together with meticulous exploration and the use of complementary imaging techniques, are essential in order to establish a correct diagnosis. A broad range of treatment options are available for supraspinatus tendinopathy. The nonsurgical alternatives include the modification of patient activities, the prescription of nonsteroidal antiinflammatory drugs, physiotherapy and different physical therapies, and a range of infiltrations. Surgery is reserved for concrete patients when the more conservative options have been exhausted and/or have failed. Acromioplasty is not an adequate alternative for the management of these patients. The aim of this review is to describe the different diagnostic and therapeutic alternatives for supraspinatus tendinopathy, given the high prevalence of this disorder.
Introduction
Painful shoulder is the third most common cause of consultation due to osteoarticular pain, after pain of the back and pain of the knee(1), and specifically rotator cuff disease is one of the most frequent causes of shoulder pain(2). The prevalence of rotator cuff disease increases after the age of 40 years(3), to the point where 50% of the population aged 60 suffers some injury that contributes to the appearance of pain and disability - with a strong impact upon the quality of life of those affected(4). Recently, an age of over 50 years, diabetes and activities involving raising the arm above the level of the head have been identified as risk factors for rotator cuff tendinopathy(5). The main aim of this review is to describe the different diagnostic and therapeutic alternatives for supraspinatus tendinopathy, which we consider important given the high prevalence of this disorder.
Pathophysiology
Traditionally, the appearance of rotator cuff disease has been described as a progressive disorder that initially manifests as acute tendinitis, continues as tendinosis with tissue degeneration and partial ruptures, and ultimately results in full thickness rupture(6). The terms tendinitis and tendinosis represent different stages of tendinopathy. Tendinitis refers to acute or chronic pain associated by definition to inflammation, even if the histological studies show an absence or only minimal presence of inflammatory cells(7). Tendinosis in turn refers to degenerative disease with or without inflammation (Figure 1). Tendinopathy is the term used to describe a clinical condition characterized by pain and functional alteration of the tendons of the rotator cuff, without specification of the concrete causal mechanism involved(8).
The etiology of rotator cuff tendinopathy is multifactorial and comprises both extrinsic and intrinsic mechanisms(9).
The extrinsic mechanisms result in compression of the bursal side of the tendon of the supraspinatus (external impingement) or of the articular side (internal impingement), and include anatomical factors, biomechanical factors, and the combination of both. The presence of anatomical variants such as acromial osteophytes, ossification of the insertion of the coracoacromial ligament, or different acromial morphologies can contribute to the appearance of tendinopathy(10), though their true role in the development of tendinopathy is limited: the different acromial morphologies as described by Neer et al.(11) and Bigliani et al.(12) show a similar incidence in individuals with no tendon alterations, and do not change with age. Furthermore, acromial osteophytes and calcification of the coracoacromial ligament that appear associated to tendinopathy, but are not a cause of the latter, seem rather to be consequences of the extrinsic compression. The role played by lateralisation of the acromion, measured with the acromion index(13), or the relationship between such lateralization and the craniocaudal inclination of the surface of the glenoid cavity, measured by the critical shoulder angle(14), are subject to debate - though it seems clear that those individuals with high acromion index values and/or critical shoulder angles suffer more supraspinatus tendinopathies and more ruptures of this tendon(15). In contrast, the development of these disorders is clearly influenced by the presence of biomechanical alterations such as scapulothoracic dyskinesia(16,17), muscle dysfunction, reduced elasticity of the pectoralis minor or postural disorders causing more or less dynamic alterations of the position of the scapula and acromion, and which produce compression upon the tendon and result in tendinopathy.
The intrinsic mechanisms are directly related to the histological structure of the tendon, its vascularization and the distribution of forces within its fibres, as well as to the changes that occur under these circumstances in the course of aging. There are zones in which mechanical overload, associated to poor vascularization with limited regenerative capacity, give rise to type I collagen degradation, with an increase in the proportion of type III collagen and organizational alteration of type IV collagen(18,19). This in turn worsens the mechanical properties of the tendon in response to tension and friction forces, and consequently gives rise to the appearance of rupture zones that can progress in response to overloading the adjacent zones.
Thus, the disorders included under the term tendinopathy comprise tendinitis, tendinosis and partial thickness lesions. Full thickness lesions fall outside the scope of this article. With regard to calcifying supraspinatus tendinopathy, we will address it as an entity in its own right, for although it constitutes an example of tendinopathy as such, the mechanism underlying its appearance has not been firmly established to date - with several hypotheses having been proposed(20) - and its imaging diagnosis moreover differs from that of the rest of the conditions.
Diagnosis
Meticulous exploration, the existence of clear lesion mechanisms, and the presence of consistent imaging findings, are essential in order to establish a correct diagnosis. It is not the objective of the present study to review exploration of the shoulder girdle. Ultrasound, magnetic resonance imaging (MRI) and MRI arthrography (arthroMRI) offer useful information on the morphological alterations that appear during the development of tendinopathy, and thus may be of help in selecting the most adequate treatment strategy(21).
Ultrasound diagnosis
One of the aspects that has changed most in relation to the use of imaging tests is the increased use of ultrasound systems outside the Radiology Department as a complement to physical examination, for example, in the field of sports medicine or traumatology(22). The fact that the results of ultrasound exploration are dependent upon the operator implies that due training is required in order to secure reliable and useful findings. The advantages of ultrasound include its cost, the safety of the procedure, and the fact that it is available in practically any medical centre. In addition, the technique can be performed simply and quickly with comparison against the healthy extremity, exploration can be made on a dynamic basis, and inter-observer correlation is excellent for many shoulder disorders. Furthermore, ultrasound-guided infiltrations are more precise than infiltrations guided by anatomical references(23),and we can perform ultrasound-guided therapeutic procedures such as needling and lavage ("barbotage") in patients with calcific tendinitis(24).
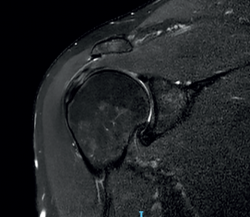
To ensure good ultrasound assessment of the posterosuperior cuff (Figure 2), we instruct the patient to place his or her arm behind the back, with the hand trying to touch the lower vertex of the scapula (Crass position). If the patient is unable to reach or keep this position during the exploration, he or she should be instructed to place the palm of the hand on the ipsilateral anterosuperior iliac crest with the elbow flexed (modification of the Crass position or Middleton position)(25). It is important to explore the tendon to its most anterior margin, as this is a frequent location of symptomatic lesions. For this purpose, in some cases the elbow must be displaced backwards, eliminating the last degrees of internal rotation.
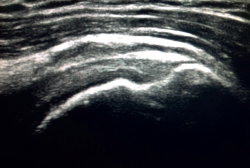
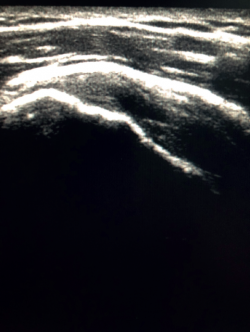
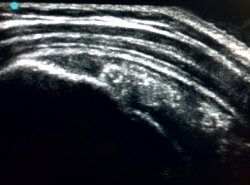
The characteristic ultrasound image of tendinosis consists of local or diffuse thickening accompanied by a hypoechogenic and heterogeneous appearance of the tendon (Figure 3). A fine hypoechoic line over 2 mm in thickness between the tendon of the supraspinatus and the subdeltoid adipose tissue corresponds to subacromial-subdeltoid bursitis, and the presence of fluid in the bursa is related to a high probability of partial or complete injury of the tendon of the supraspinatus muscle(26). In order to achieve greater diagnostic accuracy and distinguish between an inflammatory process and a normal small amount of fluid in the bursa, we should take into account that bursitis, tenosynovitis and tendinitis are characterized by the presence of hyperechoic zones with areas of enhanced flow in the Doppler ultrasound study. Both bursal and articular partial ruptures appear as a hypoechoic discontinuity in the tendon that does not vary on modifying the inclination of the ultrasound probe in either the long axis of the tendon nor its short axis(19). This latter aspect is important in order not to confuse partial lesions with anisotropic phenomena, which are a change in tissue behaviour according to the ultrasound angle of incidence(27) (Figure 4).
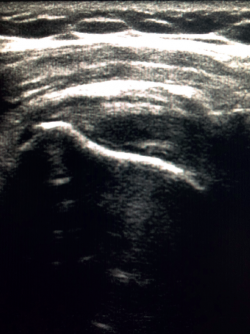
Calcific tendinitis offer different images depending on the stage of the disorder. Thus, hyperechoic, well defined deposits with clearly defined acoustic shadowing are indicative of calcific tendinitis in the formation stage. In the resting phase, the deposits appear hyperechoic, arc-shaped and with a weak acoustic shadow, while in the resorptive phase the deposits appear fragmented or nodular, with scarce acoustic shadowing (Figure 5). Furthermore, in the resorptive phase, Doppler ultrasound evidences increased vascularization around the calcifications(28).
Sonoelastography is a variant of ultrasound exploration that measures tissue elasticity in real time. It is based on the principle that soft tissue compression causes tissue displacement, and, in this regard, more rigid tissues displace less than more lax tissues(29). Such displacement is calculated in real time and is displayed on the screen of the ultrasound system as a colour scale. Therefore, sonoelastography can be used to diagnose changes in tendon ultrasound structure such as edema, tissue degeneration or partial lesions of a very small size that may go undetected by conventional ultrasound(30,31).
Magnetic resonance imaging diagnosis
Magnetic resonance imaging is a noninvasive diagnostic technique widely used in clinical practice for evaluating musculoskeletal disease conditions, and specifically for the study of the shoulder rotator cuff. It offers high sensitivity and specificity in application to cuff lesions, with great interobserver reproducibility, provided the technique is performed by shoulder experts or experienced radiologists(32).
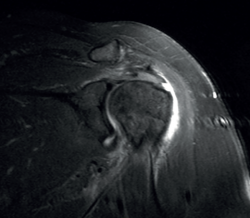
The characteristic MRI image of tendinosis consists of a focal or intra-substance zone of intermediate signal intensity in T1-weighted imaging which does not disappear in T2 sequencing and does not reach fluid signal intensity in T2-weighted imaging (Figure 6). We can sometimes observe diffuse or focal thickening of the tendon, without evidence of rupture(33). Tendinosis may prove difficult to distinguish from early-stage partial rupture of the tendon.
A lesion is classified as being of partial thickness if an abnormal fluid signal intensity in T2 sequencing is seen to extend through a portion of the tendon without affecting its full thickness (Figure 7). These may be bursal or articular lesions, though the latter are more common. There is a possibility that the thickness of the partial lesion may be occupied by scar or granulation tissue, thereby complicating the diagnosis. It has been suggested that the use of arthroMRI in the ABER (abduction + external rotation) position can improve the diagnosis and the typing of these injuries(34). According to Ellman(35), partial thickness lesions can be classified into three grades: grade I (lesion depth < 3 mm), grade II (depth 3-6 mm) and grade III (depth > 6 mm). Taking into account that a healthy supraspinatus measures between 10-12 mm in thickness, grade II lesions affect over 50% of this thickness. Intra-substance lesions are characterized by a fluid-type signal in the thickness of the tendon, without extending to the bursa or joint space.
The so-called sentinel cysts(36) are intramuscular cysts formed by a fluid collection that leaks from the joint space through a defect in the rotator cuff, and which are suggestive of damage to the latter through delamination.
Treatment
The management of patients with supraspinatus tendinopathy should be fundamented upon patient education, reduction of pain, control of mechanical loading of the tendon (under- or overloaded), and prevention of relapses(37).
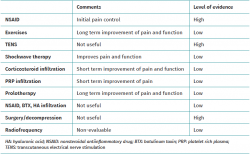
A broad range of treatment modalities for supraspinatus tendinopathy have been described, depending on the specific disease condition involved, patient age, level of activity, symptoms, degree of dysfunction, and the findings of the physical examination and imaging studies. The nonsurgical options include the modification of patient activities, the prescription of nonsteroidal antiinflammatory drugs (NSAIDs), physiotherapy and different physical therapies, and a range of infiltrations. Surgery is generally reserved for concrete patients or when the more conservative options have been exhausted and/or have failed. A summary of all the treatment modalities is provided in Table 1.
Nonsurgical options
The prescription of oral NSAIDs in both the early stage and in the chronic phase of any painful disorder is a widespread practice in our society. However, its usefulness in modifying tendinopathy has not been clearly demonstrated. In the particular case of supraspinatus tendinopathy, the presence of inflammation both in the tendon and in the bursa appears to be a feature in the early stages of tendon disease. Selective NSAIDs may inhibit cell proliferation and the synthesis of proinflammatory factors(38,39). On the other hand, NSAIDs do not induce this same response at cell level in the case of tenocytes obtained from biopsy in individuals with tendinopathy(40). In sum, NSAIDs may be useful in the early stages for the management of pain and inflammation. However, their use does not modify the clinical course or treat the tendon disease.
Manual therapy in turn comprises movement of the joints and other structures induced by a healthcare professional (e.g., physiotherapist). Such exercises include any intentional movement of a joint, muscle contraction, or prescribed patient activity. The aim of both treatments is to alleviate pain, increase the range of motion of the joints, and improve strength and function. A Cochrane review published in 2016(41) found only one article of sufficient quality comparing these treatments versus placebo. The rest of the identified publications lacked the quality needed to draw firm conclusions. Bennell(42), evaluated manual therapy associated to a home exercise protocol and found no differences in the improvement of either pain or function after 10 weeks of treatment versus placebo. Other reviews(43) have pointed out the low level of evidence on the effect of these treatments. However, they do seem able to improve pain, with no clear influence upon joint function.
Home exercise protocols generally have been regarded as basic treatment for patients with supraspinatus tendinopathy(44,45), attributing the improvement achieved to their capacity to generate structural changes in the tendon(46). This appears to be more related to the prescription of heavy-slow resistance training exercises(47). Such improvement has also been associated to changes at both central and peripheral nervous system levels, produced by the physical activity(47). Heron(48), in a clinical study evaluating different models of open chain, closed hip or range of motion exercises, reported a favourable influence of all of them in terms of pain and function, with no differences among the different exercise protocols, and recommended the use of combinations of such protocols.
Apart from the intrinsic factors that may generate supraspinatus tendinopathy, it seems clear that we must monitor and observe the extrinsic factors that generate impingement. Within this group, one of the main causes is scapulothoracic dyskinesia, which can be specifically managed based on a specific and adequate exercise protocol(49).
Transcutaneous electrical nerve stimulation (TENS) is a technique commonly used in physiotherapy to treat musculoskeletal pain. It has been postulated that TENS exerts its analgesic effect through peripheral activation of the afferent A-beta fibres responsible for blocking pain transmission (gate control theory). However, its usefulness in managing supraspinatus tendinopathy has not been demonstrated. In two available reviews - a Cochrane review from 2016(50) and another review by Desmeules et al.(51), no studies of sufficient quality were identified to allow the recommendation of TENS. In this respect, other types of treatments shown to offer greater efficacy were advised.
The use of LASER (light amplification by stimulated emission of radiation), comprising infrared light therapy, could offer some benefit for the short-term control of pain, as evidenced by a Cochrane review(50).
In turn, shockwave therapy may be effective in treating calcific supraspinatus tendinopathy, as well as tendinopathy in other anatomical locations, associated to the stimulation of neovascularization and early growth factor release at the tendon-bone junction(52). Few studies have evaluated its application to non-calcific supraspinatus tendinopathy. Galasso et al.(53) used the technique in 20 patients in a randomized study versus placebo, employing a two-session protocol with low-grade shockwaves. After three months they observed significant improvement according to the Constant scale in the treated group versus the control/placebo group. Chou et al.(54) in turn evaluated a group of patients including "throwing above head level" athletes subjected to one or two shockwave sessions. In both groups, athletes and non-athletes, a healing rate of about 50% was recorded among the patients after one year of follow-up. Considering that shockwave therapy produces few complications (pain during the sessions being the main complaint), its use is adequate as an option prior to other more aggressive treatments.
Different types of infiltrations are available as treatment or as a complement to other therapies in patients with supraspinatus tendinopathy. In this regard, there have been descriptions of infiltrations with corticosteroids, NSAIDs, hyaluronic acid (HA), botulinum toxin (BTX), platelet rich plasma (PRP), and prolotherapy (infiltration of hypertonic dextrose)(55).
Of all these options, corticosteroids (methylprednisolone, triamcinolone, betamethasone, dexamethasone) are the most widely used agents - a somewhat questionable practice, since tendinopathy is a degenerative process, not an inflammatory disorder. However, in vitro studies evidence that corticosteroids exert an effect upon the tendon and surrounding connective tissue, inhibiting the production of granulation tissue, extracellular matrix and collagen, associated to their antiinflammatory activity(56). It seems that this positive effect is only observed over the short term(57), and in vitro studies in cells have found corticosteroids to exert deleterious effects upon cell viability and proliferation, and upon the mechanical characteristics of the tendon(58). This effect demonstrated in vitro has not been correlated to the clinical outcomes, since none of the published reviews describe tendon rupture events - only the usual complications in the form of facial flushing, pain and skin pigmentation changes(56).
With regard to the use of PRP, a first aspect that must be mentioned is the great variability in the methods used to produce such plasma, yielding similar but not identical products, as well as the diversity of the treatment and application protocols used. This results in great heterogeneity among the published studies, making it difficult to draw evaluable results from the different reviews. Lin et al.(55), in their systematic review, concluded that a positive effect is observed with subacromial PRP use over the long term (more than 24 weeks) - no such effect having been observed over either the short nor the long term in other also recent reviews(59,60). It is true that PRP has been proposed by some authors as a corticosteroid substitute by virtue of its initial analgesic effect - though no differences in effectiveness between the two treatments have been recorded - and in view of the possibility of obviating the potential complications associated with corticosteroid use(61).
In vitro studies with tenocytes involving the concomitant use of PRP and corticosteroids(62) have found no alterations in the antiinflammatory effect of corticosteroids - though in contrast their deleterious effects upon the tenocytes was inhibited, with PRP inducing the synthesis of type I collagen and reducing cell apoptosis. The true usefulness of this treatment therefore remains open to doubt.
Prolotherapy, or the injection of hypertonic dextrose, is based on the theory that an irritant agent induces local inflammation within the tendon, allowing repair or scar formation(63). With regard to the application of this treatment to supraspinatus tendinopathy, most of the existing evidence is based on small retrospective series. Catapano et al.(64), in a recent systematic review, underscored the variability of the treatment models, with intra-tendon or enthesis infiltration, as well as of the control groups, corticosteroids, physiotherapy or placebo use. In any case, the authors concluded that hypertonic dextrose appears to exert a beneficial effect that seems to be more manifest with repeated enthesis infiltrations. Bertrand et al.(65) carried out a randomized clinical study of 73 patients, comparing hypertonic dextrose infiltration versus saline solution infiltration in the enthesis, and the infiltration of saline solution in the bursa. Hypertonic dextrose resulted in significantly greater improvement of the pain and patient satisfaction than the other two treatments, and the effects were also more lasting over time (up to 9 months). This finding, associated to the benefits described in other tendinopathies(63), suggests that prolotherapy may offer long term effectiveness in the management of supraspinatus tendinopathy(55), though the most suitable model and mode of use remain to be defined.
In relation to infiltrations with NSAIDs, the existing data do not show them to be superior to the use of corticosteroids(66,67). With regard to BTX, its use has been advocated in shoulder pain in hemiplegic patients(68). Hyaluronic acid has not been found to be of benefit in patients without osteoarthritis(55).
In sum, it can be concluded that there is moderate evidence that corticosteroid infiltrations offer benefit in controlling pain over the short term, and that prolotherapy may be a promising option for securing long-term improvement. In any case, in order to ensure the adequate use of these treatments, ultrasound-guided infiltration allows more precise localization of the administration site, since the utilization of a "blind" infiltration method is associated to a high percentage of drug delivery in undesired locations. Correct targeting of the subacromial space has been reported in 70% of the cases, with location in the deltoid muscle in 21%, in the glenohumeral joint in 4%, and at subcutaneous level in 5% of the cases(63).
Surgical options
Subacromial decompression as treatment for chronic supraspinatus tendinopathy has been widely recommended and used. Acromioplasty has been compared versus different treatments such as placebo, physical therapy, corticosteroid infiltrations and isolated bursectomy, with no differences being observed with respect to any of them(69). Ketola et al. recorded no differences between surgery in the form of arthroscopic subacromial decompression and conservative management after 12 years of follow-up in patients with subacromial syndrome. It is therefore not possible to recommend acromioplasty as treatment for supraspinatus tendinopathy(70). In addition, the role of "lateral" acromioplasty to modify the lateral coverage of the acromion is subject to much debate(71).
Another surgical option is radiofrequency microtenotomy(72). The available studies(72,73) compare radiofrequency versus subacromial decompression, with similar results after two years of follow-up. Radiofrequency is described as offering advantages over acromioplasty in that it avoids the resection of bone and the coracoacromial ligament, which may be part of the deleterious effects of subacromial decompression.
In relation to partial damage to the rotator cuff, there is currently no scientific evidence as to which treatment is best for such symptomatic lesions. This is because it is difficult to compare the results of the different therapeutic alternatives, due to the heterogeneity of the lesion under study and of the tools used to evaluate the results of the different treatments(74). Arthroscopic debridement with or without acromioplasty in application to Ellman grade I/II lesions(35) and the different repair techniques (trans-tendon or completing rupture) in the case of Ellman grade III lesions are the most widely used surgical techniques.
With regard to calcifying supraspinatus tendinitis(28), the first line of treatment consists of rehabilitation, the prescription of NSAIDs and corticosteroid infiltrations - though the failure rate is close to 30%, since none of these alternatives eliminate the calcium deposits. The probability of failure is greater in the case of calcifications that are large, bilateral, and extend beyond the medial margin of the acromion or are located under its anterior third. Calcifications that are in the forming stage and are dense and homogeneous (Gärtner and Heyer type I) appear to benefit from shockwave therapy, while those that are in the resting or resorptive phase (Gärtner and Heyer types II and III), and are heterogeneous, segmented, and poorly delimited, with a fluid content, can benefit from percutaneous lavage under ultrasound control, also known as "barbotage"(75).
Conclusions
Supraspinatus tendinopathy is a common cause of shoulder dysfunction and pain, and comprises conditions ranging from tendinitis to partial rupture. Ultrasound in expert or trained hands is an excellent diagnostic tool in such situations - its main advantages being great accessibility, the possibility of performing comparative explorations versus the healthy extremity, the possibility of dynamic explorations, and ultrasound-guided therapy. Magnetic resonance imaging is another widely used noninvasive diagnostic technique, offering high sensitivity and specificity, as well as great imaging quality and information regarding concomitant lesions. The treatments described for supraspinatus tendinopathy are highly varied, and the therapeutic decision will depend on the type of patient and the moment or type of lesion involved.
Figuras
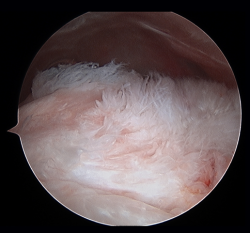
Figure 1. Arthroscopic view from the posterior portal in a case of severe supraspinatus tendinosis of a left shoulder.
Figure 4. Anisotropy at the insertion of the tendon of the supraspinatus muscle. Not to be confused with partial thickness injury.
Figure 5. Ultrasound view of calcific supraspinatus tendinopathy, corresponding to Gärtner and Heyer type III.
Tablas
Información del artículo
Cita bibliográfica
Autores
Santos Moros Marco
Servicio de Traumatología. Hospital MAZ. Zaragoza
Cirugía Ortopédica y Traumatología. Unidad de Miembro Superior. Hospital MAZ Zaragoza. Arthrosport Zaragoza
Editor asociado de REACA
Servicio de Traumatología y Cirugía Ortopédica. Hospital de la Mutua MAZ. Zaragoza
Jorge Díaz Heredia
Cirugía Ortopédica y Traumatología. Unidad de Hombro y Codo. Hospital Universitario Ramón y Cajal. Madrid
Clínica La Antigua. Guadalajara
Editor asociado de REACA
Miguel Ángel Ruiz Ibán
Director de REACA
Cirugía Ortopédica y Traumatología. Unidad de Hombro y Codo. Hospital Universitario Ramón y Cajal. Madrid
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved studies in humans or in animals.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Brox JI. Regional musculoskeletal conditions: shoulder pain. Best Pract Res Clin Rheumatol. 2003;17(1):33-56.
-
2Van der Windt DA, Koes BW, Boeke AJ, Deville W, De Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract. 1996;46(410):519-23.
-
3Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8(4):296-9.
-
4MacDermid JC, Ramos J, Drosdowech D, Faber K, Patterson S. The impact of rotator cuff pathology on isometric and isokinetic strength, function, and quality of life. J Shoulder Elbow Surg. 2004;13(6):593-8.
-
5Leong HT, Fu SC, He X, Oh JH, Yamamoto N, Hang S. Risk factors for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2019;51(9):627-37.
-
6Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983(173):70-7.
-
7Fukuda H, Hamada K, Yamanaka K. Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop Relat Res. 1990(254):75-80.
-
8Fredberg U, Stengaard-Pedersen K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand J Med Sci Sports. 2008;18(1):3-15.
-
9Seitz AL, McClure PW, Finucane S, Boardman ND 3rd, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon). 2011;26(1):1-12.
-
10Soslowsky LJ, Thomopoulos S, Esmail A, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30(8):1057-63.
-
11Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
-
12Bigliani LU, Morrison DS, April EW. The morphology of the acromion and its relationship to rotator cuff tears. Ortho Trans. 1986;10
-
13Nyffeler RW, Werner CM, Sukthankar A, Schmid MR, Gerber C. Association of a large lateral extension of the acromion with rotator cuff tears. J Bone Joint Surg Am. 2006;88(4):800-5.
-
14Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: a radiological study of the critical shoulder angle. Bone Joint J. 2013;95-B(7):935-41.
-
15Smith GCS, Liu V, Lam PH. The Critical Shoulder Angle Shows a Reciprocal Change in Magnitude When Evaluating Symptomatic Full-Thickness Rotator Cuff Tears Versus Primary Glenohumeral Osteoarthritis as Compared With Control Subjects: A Systematic Review and Meta-analysis. Arthroscopy. 2020;36(2):566-75.
-
16Roche SJ, Funk L, Sciascia A, Kibler WB. Scapular dyskinesis: the surgeon's perspective. Shoulder Elbow. 2015;7(4):289-97.
-
17McClure PW, Michener LA, Karduna AR. Shoulder function and 3-dimensional scapular kinematics in people with and without shoulder impingement syndrome. Phys Ther. 2006;86(8):1075-90.
-
18Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53(6):359-66.
-
19Thakkar D, Grant TM, Hakimi O, Carr AJ. Distribution and expression of type VI collagen and elastic fibers in human rotator cuff tendon tears. Connect Tissue Res. 2014;55(5-6):397-402.
-
20Suzuki K, Potts A, Anakwenze O, Singh A. Calcific tendinitis of the rotator cuff: management options. J Am Acad Orthop Surg. 2014;22(11):707-17.
-
21Roy JS, Braen C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. 2015;49(20):1316-28.
-
22Harmon KG, O'Connor FG. Musculoskeletal ultrasound: taking sports medicine to the next level. Br J Sports Med. 2010;44(16):1135-6.
-
23Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-9.
-
24Yoo JC, Koh KH, Park WH, Park JC, Kim SM, Yoon YC. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elbow Surg. 2010;19(4):596-600.
-
25Zheng F, Wang H, Gong H, Fan H, Zhang K, Du L. Role of Ultrasound in the Detection of Rotator-Cuff Syndrome: An Observational Study. Med Sci Monit. 2019;25:5856-63.
-
26Jacobson JA, Lancaster S, Prasad A, van Holsbeeck MT, Craig JG, Kolowich P. Full-thickness and partial-thickness supraspinatus tendon tears: value of US signs in diagnosis. Radiology. 2004;230(1):234-42.
-
27Singh JP. Shoulder ultrasound: What you need to know. Indian J Radiol Imaging. 2012;22(4):284-92.
-
28Merolla G, Singh S, Paladini P, Porcellini G. Calcific tendinitis of the rotator cuff: state of the art in diagnosis and treatment. J Orthop Traumatol. 2016;17(1):7-14.
-
29Yanagisawa O, Niitsu M, Kurihara T, Fukubayashi T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: a feasibility study. Clin Radiol. 2011;66(9):815-9.
-
30Vasishta A, Kelkar A, Joshi P, Hapse R. The value of sonoelastography in the diagnosis of supraspinatus tendinopathy-a comparison study. Br J Radiol. 2019;92(1095):20180951.
-
31Seo JB, Yoo JS, Ryu JW. Sonoelastography findings of supraspinatus tendon in rotator cuff tendinopathy without tear: comparison with magnetic resonance images and conventional ultrasonography. J Ultrasound. 2015;18(2):143-9.
-
32Jain NB, Collins J, Newman JS, Katz JN, Losina E, Higgins LD. Reliability of magnetic resonance imaging assessment of rotator cuff: the ROW study. PM R. 2015;7(3):245-54 e243; quiz 254.
-
33Opsha O, Malik A, Baltazar R, et al. MRI of the rotator cuff and internal derangement. Eur J Radiol. 2008;68(1):36-56.
-
34Herold T, Bachthaler M, Hamer OW, et al. Indirect MR arthrography of the shoulder: use of abduction and external rotation to detect full- and partial-thickness tears of the supraspinatus tendon. Radiology. 2006;240(1):152-60.
-
35Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990(254):64-74.
-
36Sanders TG, Tirman PF, Feller JF, Genant HK. Association of intramuscular cysts of the rotator cuff with tears of the rotator cuff: magnetic resonance imaging findings and clinical significance. Arthroscopy. 2000;16(3):230-5.
-
37Lewis JS. Rotator cuff tendinopathy: a model for the continuum of pathology and related management. Br J Sports Med. 2010;44(13):918-23.
-
38Riley GP, Cox M, Harrall RL, Clements S, Hazleman BL. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J Hand Surg Br. 2001;26(3):224-8.
-
39Tsai WC, Tang FT, Hsu CC, Hsu YH, Pang JH, Shiue CC. Ibuprofen inhibition of tendon cell proliferation and upregulation of the cyclin kinase inhibitor p21CIP1. J Orthop Res. 2004;22(3):586-91.
-
40Heinemeier KM, Ohlenschlaeger TF, Mikkelsen UR, et al. Effects of anti-inflammatory (NSAID) treatment on human tendinopathic tissue. J Appl Physiol (1985). 2017;123(5):1397-405.
-
41Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016(6):CD012224.
-
42Bennell K, Coburn S, Wee E, et al. Efficacy and cost-effectiveness of a physiotherapy program for chronic rotator cuff pathology: a protocol for a randomised, double-blind, placebo-controlled trial. BMC Musculoskelet Disord. 2007;8:86.
-
43Desjardins-Charbonneau A, Roy JS, Dionne CE, Fremont P, MacDermid JC, Desmeules F. The efficacy of manual therapy for rotator cuff tendinopathy: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2015;45(5):330-50.
-
44Steuri R, Sattelmayer M, Elsig S, et al. Effectiveness of conservative interventions including exercise, manual therapy and medical management in adults with shoulder impingement: a systematic review and meta-analysis of RCTs. Br J Sports Med. 2017 Sep;51(18):1340-7.
-
45Littlewood C, Ashton J, Chance-Larsen K, May S, Sturrock B. Exercise for rotator cuff tendinopathy: a systematic review. Physiotherapy. 2012;98(2):101-9.
-
46Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409-16.
-
47Drew BT, Smith TO, Littlewood C, Sturrock B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):966-72.
-
48Heron SR, Woby SR, Thompson DP. Comparison of three types of exercise in the treatment of rotator cuff tendinopathy/shoulder impingement syndrome: a randomized controlled trial. Physiotherapy. 2017;103(2):167-73.
-
49Panagiotopoulos AC, Crowther IM. Scapular dyskinesia, the forgotten culprit of shoulder pain and how to rehabilitate. SICOT J. 2019;5:29.
-
50Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016(6):CD012225.
-
51Desmeules F, Boudreault J, Roy JS, Dionne CE, Fremont P, MacDermid JC. Efficacy of transcutaneous electrical nerve stimulation for rotator cuff tendinopathy: a systematic review. Physiotherapy. 2016;102(1):41-9.
-
52Wang CJ, Wang FS, Yang KD, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21(6):984-9.
-
53Galasso O, Amelio E, Riccelli DA, Gasparini G. Short-term outcomes of extracorporeal shock wave therapy for the treatment of chronic non-calcific tendinopathy of the supraspinatus: a double-blind, randomized, placebo-controlled trial. BMC Musculoskelet Disord. 2012;13:86.
-
54Chou WY, Wang CJ, Wu KT, Yang YJ, Cheng JH, Wang SW. Comparative outcomes of extracorporeal shockwave therapy for shoulder tendinitis or partial tears of the rotator cuff in athletes and non-athletes: Retrospective study. Int J Surg. 2018;51:184-90.
-
55Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative Effectiveness of Injection Therapies in Rotator Cuff Tendinopathy: A Systematic Review, Pairwise and Network Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil. 2019;100(2):336-49 e315.
-
56Paavola M, Kannus P, Jarvinen TA, Jarvinen TL, Jozsa L, Jarvinen M. Treatment of tendon disorders. Is there a role for corticosteroid injection? Foot Ankle Clin. 2002;7(3):501-13.
-
57Lin KM, Wang D, Dines JS. Injection Therapies for Rotator Cuff Disease. Orthop Clin North Am. 2018;49(2):231-9.
-
58Dean BJ, Lostis E, Oakley T, Rombach I, Morrey ME, Carr AJ. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43(4):570-6.
-
59Hurley ET, Lim Fat D, Moran CJ, Mullett H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2018:363546517751397.
-
60Filardo G, Di Matteo B, Kon E, Merli G, Marcacci M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1984-99.
-
61Carr JB 2nd, Rodeo SA. The role of biologic agents in the management of common shoulder pathologies: current state and future directions. J Shoulder Elbow Surg. 2019;28(11):2041-52.
-
62Jo CH, Lee SY, Yoon KS, Shin S. Effects of Platelet-Rich Plasma With Concomitant Use of a Corticosteroid on Tenocytes From Degenerative Rotator Cuff Tears in Interleukin 1beta-Induced Tendinopathic Conditions. Am J Sports Med. 2017;45(5):1141-50.
-
63Reeves KD, Sit RW, Rabago DP. Dextrose Prolotherapy: A Narrative Review of Basic Science, Clinical Research, and Best Treatment Recommendations. Phys Med Rehabil Clin N Am. 2016;27(4):783-823.
-
64Catapano M, Zhang K, Mittal N, Sangha H, Onishi K, de Sa D. Effectiveness of Dextrose Prolotherapy for Rotator Cuff Tendinopathy: A Systematic Review. PM R. 2020;12(3):288-300.
-
65Bertrand H, Reeves KD, Bennett CJ, Bicknell S, Cheng AL. Dextrose Prolotherapy Versus Control Injections in Painful Rotator Cuff Tendinopathy. Arch Phys Med Rehabil. 2016;97(1):17-25.
-
66Karthikeyan S, Kwong HT, Upadhyay PK, Parsons N, Drew SJ, Griffin D. A double-blind randomised controlled study comparing subacromial injection of tenoxicam or methylprednisolone in patients with subacromial impingement. J Bone Joint Surg Br. 2010;92(1):77-82.
-
67Aksakal M, Ermutlu C, Ozkaya G, Ozkan Y. Lornoxicam injection is inferior to betamethasone in the treatment of subacromial impingement syndrome: a prospective randomized study of functional outcomes. Orthopade. 2017;46(2):179-85.
-
68Wu T, Fu Y, Song HX, Ye Y, Dong Y, Li JH. Effectiveness of Botulinum Toxin for Shoulder Pain Treatment: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2015;96(12):2214-20.
-
69Vandvik PO, Lahdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ. 2019;364:l294.
-
70Ketola S, Lehtinen JT, Arnala I. Arthroscopic decompression not recommended in the treatment of rotator cuff tendinopathy: a final review of a randomised controlled trial at a minimum follow-up of ten years. Bone Joint J. 2017;99-B(6):799-805.
-
71Meislin R. Editorial Commentary: #Fakeradiographicangle-Critical Shoulder Angle, Like Acromioplasty, May Not Be Critical. Arthroscopy. 2019;35(11):3144-5.
-
72Al-Ani Z, Jacobsen EW, Kartus JT, Knutsen G, Meknas K. Radiofrequency microtenotomy: a promising method for treatment of rotator cuff tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2019;27(12):3856-63.
-
73Taverna E, Battistella F, Sansone V, Perfetti C, Tasto JP. Radiofrequency-based plasma microtenotomy compared with arthroscopic subacromial decompression yields equivalent outcomes for rotator cuff tendinosis. Arthroscopy. 2007;23(10):1042-51.
-
74Oliva F, Piccirilli E, Bossa M, et al. I.S.Mu.L.T - Rotator Cuff Tears Guidelines. Muscles Ligaments Tendons J. 2015;5(4):227-63.
-
75Zhang T, Duan Y, Chen J, Chen X. Efficacy of ultrasound-guided percutaneous lavage for rotator cuff calcific tendinopathy: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(21):e15552.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Tendon injury: from eternally forgotten to trendy disease
- Physiology and mechanobiology of tendon and muscle tissue
- Patellar tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Pathophysiology, diagnosis and treatment of Achilles tendinopathy
- Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
- Update on the diagnosis and treatment of quadriceps muscle injuries
- Management of triceps surae muscle injuries in young adult and middle-aged athletes: a narrative literature review
- Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
- Inverted cyclops
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.